Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials
- PMID: 23433102
- PMCID: PMC3598551
- DOI: 10.1186/1475-2875-12-70
Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials
Abstract
Background: Pyronaridine-artesunate (PA) is indicated for the treatment of acute uncomplicated Plasmodium falciparum and Plasmodium vivax malaria.
Methods: Individual patient data on safety outcomes were integrated from six randomized clinical trials conducted in Africa and Asia in patients with microscopically confirmed P. falciparum (five studies) or P. vivax (one study) malaria. Efficacy against P. falciparum was evaluated across three Phase III clinical trials.
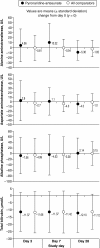
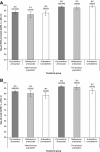
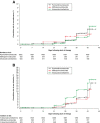
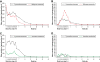
Results: The safety population included 2,815 patients randomized to PA, 1,254 to comparators: mefloquine + artesunate (MQ + AS), artemether-lumefantrine (AL), or chloroquine. All treatments were generally well tolerated. Adverse events occurred in 57.2% (1,611/2,815) of patients with PA versus 51.5% (646/1,254) for comparators, most commonly (PA; comparators): headache (10.6%; 9.9%), cough (5.9%; 5.6%) and anaemia (4.5%; 2.9%). Serious averse events were uncommon for all treatments (0-0.7%). Transient increases in alanine aminotransferase and aspartate aminotransferase were observed with PA but did not lead to any clinical sequelae. For P. falciparum malaria, day-28 PCR-corrected adequate clinical and parasitological response with PA was 93.6% ([1,921/2,052] 95% CI 92.6, 94.7) in the intent-to-treat population and 98.5% ([1,852/1,880] 95% CI 98.0, 99.1) in the per-protocol population. Median parasite clearance time was 24.1 h with PA, 31.9 h with MQ + AS, and 24.0 h with AL. Median fever clearance time was 15.5 h with PA, 15.8 h with MQ + AS, and 14.0 h with AL. By day 42, P. falciparum gametocytes had declined to near zero for all treatments.
Conclusions: Pyronaridine-artesunate was well tolerated with no safety concerns with the exception of mostly mild transient rises in transaminases. Efficacy was high and met the requirements for use as first-line therapy. Pyronaridine-artesunate should be considered for inclusion in malaria treatment programmes.
Trial registration: Clinicaltrials.gov: NCT00331136; NCT00403260; NCT00422084; NCT00440999; NCT00541385; NCT01594931.
Figures




References
-
- World Health Organization. World Malaria Report. 2011. http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf.
-
- Roll Back Malaria Partnership Secretariat. World Malaria Day. 2010. Africa Update http://www.rollbackmalaria.org/ProgressImpactSeries/docs/wmd2010report-e....
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

