Histone modifications in the male germ line of Drosophila
- PMID: 23433182
- PMCID: PMC3602674
- DOI: 10.1186/1471-213X-13-7
Histone modifications in the male germ line of Drosophila
Abstract
Background: In the male germ line of Drosophila chromatin remains decondensed and highly transcribed during meiotic prophase until it is rapidly compacted. A large proportion of the cell cycle-regulated histone H3.1 is replaced by H3.3, a histone variant encoded outside the histone repeat cluster and not subject to cell cycle controlled expression.
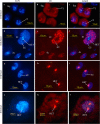
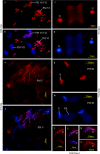
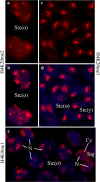
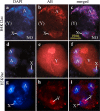
Results: We investigated histone modification patterns in testes of D. melanogaster and D. hydei. In somatic cells of the testis envelope and in germ cells these modification patterns differ from those typically seen in eu- and heterochromatin of other somatic cells. During the meiotic prophase some modifications expected in active chromatin are not found or are found at low level. The absence of H4K16ac suggests that dosage compensation does not take place. Certain histone modifications correspond to either the cell cycle-regulated histone H3.1 or to the testis-specific variant H3.3. In spermatogonia we found H3K9 methylation in cytoplasmic histones, most likely corresponding to the H3.3 histone variant. Most histone modifications persist throughout the meiotic divisions. The majority of modifications persist until the early spermatid nuclei, and only a minority further persist until the final chromatin compaction stages before individualization of the spermatozoa.
Conclusion: Histone modification patterns in the male germ line differ from expected patterns. They are consistent with an absence of dosage compensation of the X chromosome during the male meiotic prophase. The cell cycle-regulated histone variant H3.1 and H3.3, expressed throughout the cell cycle, also vary in their modification patterns. Postmeiotically, we observed a highly complex pattern of the histone modifications until late spermatid nuclear elongation stages. This may be in part due to postmeiotic transcription and in part to differential histone replacement during chromatin condensation.
Figures
Similar articles
-
Balanced spatiotemporal arrangements of histone H3 and H4 posttranslational modifications are necessary for meiotic prophase I chromosome organization.J Cell Physiol. 2024 Apr;239(4):e31201. doi: 10.1002/jcp.31201. Epub 2024 Jan 29. J Cell Physiol. 2024. PMID: 38284481
-
The localization of histone H3.3 in germ line chromatin of Drosophila males as established with a histone H3.3-specific antiserum.Chromosoma. 1997 Nov;106(6):335-47. doi: 10.1007/s004120050255. Chromosoma. 1997. PMID: 9362542
-
Chromosomal proteins in the spermatogenesis of Drosophila.Chromosoma. 2003 May;111(8):489-94. doi: 10.1007/s00412-003-0236-6. Epub 2003 Mar 28. Chromosoma. 2003. PMID: 12684823 Review.
-
Histone H3.3 variant dynamics in the germline of Caenorhabditis elegans.PLoS Genet. 2006 Jun;2(6):e97. doi: 10.1371/journal.pgen.0020097. PLoS Genet. 2006. PMID: 16846252 Free PMC article.
-
Dosage dependent gene regulation and the compensation of the X chromosome in Drosophila males.Genetica. 2003 Mar;117(2-3):179-90. doi: 10.1023/a:1022935927763. Genetica. 2003. PMID: 12723697 Review.
Cited by
-
Reduced Levels of Lagging Strand Polymerases Shape Stem Cell Chromatin.bioRxiv [Preprint]. 2024 Apr 29:2024.04.26.591383. doi: 10.1101/2024.04.26.591383. bioRxiv. 2024. Update in: Sci Adv. 2025 Feb 28;11(9):eadu6799. doi: 10.1126/sciadv.adu6799. PMID: 38746451 Free PMC article. Updated. Preprint.
-
Meiosis in the scorpion Tityus silvestris: new insights into achiasmatic chromosomes.Biol Open. 2019 May 9;8(5):bio040352. doi: 10.1242/bio.040352. Biol Open. 2019. PMID: 31072909 Free PMC article.
-
Epigenetic regulation of drosophila germline stem cell maintenance and differentiation.Dev Biol. 2021 May;473:105-118. doi: 10.1016/j.ydbio.2021.02.003. Epub 2021 Feb 18. Dev Biol. 2021. PMID: 33610541 Free PMC article. Review.
-
Male meiosis in Crustacea: synapsis, recombination, epigenetics and fertility in Daphnia magna.Chromosoma. 2016 Sep;125(4):769-87. doi: 10.1007/s00412-015-0558-1. Epub 2015 Dec 21. Chromosoma. 2016. PMID: 26685998 Free PMC article.
-
Increased levels of lagging strand polymerase α in an adult stem cell lineage affect replication-coupled histone incorporation.Sci Adv. 2025 Feb 28;11(9):eadu6799. doi: 10.1126/sciadv.adu6799. Epub 2025 Feb 28. Sci Adv. 2025. PMID: 40020063 Free PMC article.
References
-
- Weyrich A. DNA methylation, histone modification and the transcription factor dE2F1 in Drosophila. Mainz: Johannes Gutenberg-University Mainz; 2007.
-
- Kremer H, Hennig W, Dijkhof R. Chromatin organization in the male germ line of Drosophila. Chromosoma. 1986;94:147–161. doi: 10.1007/BF00288489. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

