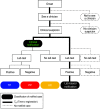
A new surveillance indicator identifying optimal timeliness and accuracy: application to the Korean National Notifiable Disease Surveillance System for 2001-2007
- PMID: 23433204
- PMCID: PMC9151359
- DOI: 10.1017/S0950268812002956
A new surveillance indicator identifying optimal timeliness and accuracy: application to the Korean National Notifiable Disease Surveillance System for 2001-2007
Abstract
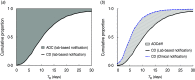
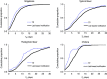
Although immediate notification of a case is crucial for epidemic control, clinicians may delay notification due to uncertainties in diagnosis, reflecting a trade-off between timeliness and the accuracy of surveillance. We assessed this trade-off for four epidemic-prone diseases that require immediate notification of suspected cases: shigellosis, typhoid fever, paratyphoid fever, and cholera in the Korean National Notifiable Disease Surveillance System data for 2001-2007. Timeliness was measured as the time to registration (T R), being the time interval from symptom onset to notification by the clinician to the local public health centre. We introduced a new index, 'time-accuracy trade-off ratio' to indicate time saved by clinical vs. laboratory-based notifications. Clinical notifications comprised 34.4% of total notifications, and these showed a shorter median T R than laboratory-based notifications (1-4 days). The trade-off ratio was greatest for shigellosis (3.3 days), and smallest for typhoid fever (0.6 days). A higher trade-off ratio provides stronger evidence for clinical notification without waiting for laboratory confirmation.
Figures
References
-
- Day F, Sutton G. General practitioner notifications of gastroenteritis and food poisoning: cause for concern. Journal of Public Health 2007; 29: 288–291. - PubMed
-
- Barrett P, Lau YK. Incompleteness of statutory notification of bacterial gastro-intestinal infection. Public Health 1997; 111: 183–185. - PubMed
-
- Clarkson JA, Fine PE. Delays in notification of infectious disease. Health Trends 1987; 19: 9–11. - PubMed
-
- World Health Organization. Communicable disease surveillance and response systems – Guide to monitoring and evaluating (http://www.who.int/csr/resources/publications/surveillance/WHO_CDS_EPR_L...). Accessed 18 March 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

