Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer
- PMID: 23433435
- PMCID: PMC3599345
- DOI: 10.1186/1471-2407-13-84
Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer
Abstract
Background: Failure of locoregional control is the main cause of recurrence in advanced head and neck cancer. This multi-center trial aims to improve outcome in two ways. Firstly, by redistribution of the radiation dose to the metabolically most FDG-PET avid part of the tumour. Hereby, a biologically more effective dose distribution might be achieved while simultaneously sparing normal tissues. Secondly, by improving patient selection. Both cisplatin and Epidermal Growth Factor Receptor (EGFR) antibodies like Cetuximab in combination with Radiotherapy (RT) are effective in enhancing tumour response. However, it is unknown which patients will benefit from either agent in combination with irradiation. We will analyze the predictive value of biological markers and (89)Zr-Cetuximab uptake for treatment outcome of chemoradiation with Cetuximab or cisplatin to improve patient selection.
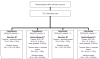
Methods: ARTFORCE is a randomized phase II trial for 268 patients with a factorial 2 by 2 design: cisplatin versus Cetuximab and standard RT versus redistributed RT. Cisplatin is dosed weekly 40 mg/m(2) for 6 weeks. Cetuximab is dosed 250 mg/m(2) weekly (loading dose 400 mg/m(2)) for 6 weeks. The standard RT regimen consists of elective RT up to 54.25 Gy with a simultaneous integrated boost (SIB) to 70 Gy in 35 fractions in 6 weeks. Redistributed adaptive RT consists of elective RT up to 54.25 Gy with a SIB between 64-80 Gy in 35 fractions in 6 weeks with redistributed dose to the gross tumour volume (GTV) and clinical target volume (CTV), and adaptation of treatment for anatomical changes in the third week of treatment.Patients with locally advanced, biopsy confirmed squamous cell carcinoma of the oropharynx, oral cavity or hypopharynx are eligible.Primary endpoints are: locoregional recurrence free survival at 2 years, correlation of the median (89)Zr-cetuximab uptake and biological markers with treatment specific outcome, and toxicity. Secondary endpoints are quality of life, swallowing function preservation, progression free and overall survival.
Discussion: The objective of the ARTFORCE Head and Neck trial is to determine the predictive value of biological markers and (89)Zr-Cetuximab uptake, as it is unknown how to select patients for the appropriate concurrent agent. Also we will determine if adaptive RT and dose redistribution improve locoregional control without increasing toxicity.ClinicalTrials.gov Identifier: NCT01504815.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

