CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors
- PMID: 23434321
- PMCID: PMC3594500
- DOI: 10.1016/j.cell.2013.01.053
CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors
Abstract
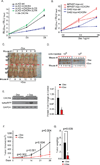
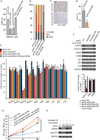
Malignant peripheral nerve sheath tumors (MPNSTs) are soft tissue sarcomas that arise in connective tissue surrounding peripheral nerves. They occur sporadically in a subset of patients with neurofibromatosis type 1 (NF1). MPNSTs are highly aggressive, therapeutically resistant, and typically fatal. Using comparative transcriptome analysis, we identified CXCR4, a G-protein-coupled receptor, as highly expressed in mouse models of NF1-deficient MPNSTs, but not in nontransformed precursor cells. The chemokine receptor CXCR4 and its ligand, CXCL12, promote MPNST growth by stimulating cyclin D1 expression and cell-cycle progression through PI3-kinase (PI3K) and β-catenin signaling. Suppression of CXCR4 activity either by shRNA or pharmacological inhibition decreases MPNST cell growth in culture and inhibits tumorigenesis in allografts and in spontaneous genetic mouse models of MPNST. We further demonstrate conservation of these activated molecular pathways in human MPNSTs. Our findings indicate a role for CXCR4 in NF1-associated MPNST development and identify a therapeutic target.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Interweaving the strands: β-catenin, an HIV co-receptor, and Schwann cell tumors.Cancer Cell. 2013 Mar 18;23(3):269-71. doi: 10.1016/j.ccr.2013.03.001. Cancer Cell. 2013. PMID: 23518344 Free PMC article.
References
-
- Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. - PubMed
-
- Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, Gobbi A, Pruneri G, Martinelli G. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res. 2002;62:3106–3112. - PubMed
-
- Bian XW, Yang SX, Chen JH, Ping YF, Zhou XD, Wang QL, Jiang XF, Gong W, Xiao HL, Du LL, et al. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery. 2007;61:570–578. discussion 578–579. - PubMed
-
- Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–3667. - PubMed
-
- Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

