A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients
- PMID: 23434567
- PMCID: PMC3888620
- DOI: 10.1136/annrheumdis-2012-202794
A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients
Abstract
Objective: To assess the pharmacodynamic effects of sifalimumab, an investigational anti-IFN-α monoclonal antibody, in the blood and muscle of adult dermatomyositis and polymyositis patients by measuring neutralisation of a type I IFN gene signature (IFNGS) following drug exposure.
Methods: A phase 1b randomised, double-blinded, placebo controlled, dose-escalation, multicentre clinical trial was conducted to evaluate sifalimumab in dermatomyositis or polymyositis patients. Blood and muscle biopsies were procured before and after sifalimumab administration. Selected proteins were measured in patient serum with a multiplex assay, in the muscle using immunohistochemistry, and transcripts were profiled with microarray and quantitative reverse transcriptase PCR assays. A 13-gene IFNGS was used to measure the pharmacological effect of sifalimumab.
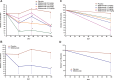
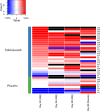
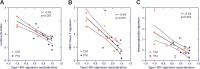
Results: The IFNGS was suppressed by a median of 53-66% across three time points (days 28, 56 and 98) in blood (p=0.019) and 47% at day 98 in muscle specimens post-sifalimumab administration. Both IFN-inducible transcripts and proteins were prevalently suppressed following sifalimumab administration. Patients with 15% or greater improvement from baseline manual muscle testing scores showed greater neutralisation of the IFNGS than patients with less than 15% improvement in both blood and muscle. Pathway/functional analysis of transcripts suppressed by sifalimumab showed that leucocyte infiltration, antigen presentation and immunoglobulin categories were most suppressed by sifalimumab and highly correlated with IFNGS neutralisation in muscle.
Conclusions: Sifalimumab suppressed the IFNGS in blood and muscle tissue in myositis patients, consistent with this molecule's mechanism of action with a positive correlative trend between target neutralisation and clinical improvement. These observations will require confirmation in a larger trial powered to evaluate efficacy.
Trial registration: ClinicalTrials.gov NCT00533091.
Keywords: Cytokines; Dermatomyositis; Polymyositis.
Figures


References
-
- Mastaglia FL, Garlepp MJ, Phillips BA, et al. Inflammatory myopathies: clinical, diagnostic and therapeutic aspects. Muscle Nerve 2003;27:407–25 - PubMed
-
- Christopher-Stine L, Plotz PH. Adult inflammatory myopathies. Best Pract Res Clin Rheumatol 2004;18:331–44 - PubMed
-
- Amato AA, Griggs RC. Treatment of idiopathic inflammatory myopathies. Curr Opin Neurol 2003;16:569–75 - PubMed
-
- Choy EH, Hoogendijk JE, Lecky B, et al. Withdrawn: immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2009;4:CD003643. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

