Characterization of the adipocyte cellular lineage in vivo
- PMID: 23434825
- PMCID: PMC3721064
- DOI: 10.1038/ncb2696
Characterization of the adipocyte cellular lineage in vivo
Abstract
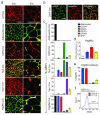
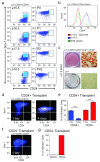
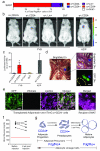
Mature adipocytes are generated through the proliferation and differentiation of precursor cells. Our previous studies identified adipocyte progenitors in white adipose tissue (WAT) as Lin(-):CD29(+):CD34(+):Sca-1(+):CD24(+) (CD24(+)) cells that are capable of generating functional WAT (ref. ). Here, we employ several Cre recombinase mouse models to identify the adipocyte cellular lineage in vivo. Although it has been proposed that white adipocytes are derived from endothelial and haematopoietic lineages, we find that neither of these lineages label white adipocytes. However, platelet-derived growth factor receptor α (PdgfRα)-Cre trace labels all white adipocytes. Analysis of WAT from PdgfRα-Cre reporter mice identifies CD24(+) and Lin(-):CD29(+):CD34(+):Sca-1(+): CD24(-) (CD24(-)) cells as adipocyte precursors. We show that CD24(+) cells generate the CD24(-) population in vivo and the CD24(-) cells express late markers of adipogenesis. From these data we propose a model where the CD24(+) adipocyte progenitors become further committed to the adipocyte lineage as CD24 expression is lost, generating CD24(-) preadipocytes. This characterization of the adipocyte cellular lineage will facilitate the study of the mechanisms that regulate WAT formation in vivo and WAT mass expansion in obesity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

