The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses
- PMID: 23435120
- PMCID: PMC4161284
- DOI: 10.1038/ni.2541
The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses
Erratum in
- Nat Immunol. 2014 Mar;15(3):305. Kaplan, Daniel H [added]
Abstract
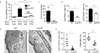
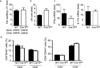
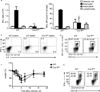
Dendritic cells (DCs) are critical in immune responses, linking innate and adaptive immunity. We found here that DC-specific deletion of the transcription factor STAT5 was not critical for development but was required for T helper type 2 (TH2), but not TH1, allergic responses in both the skin and lungs. Loss of STAT5 in DCs led to the inability to respond to thymic stromal lymphopoietin (TSLP). STAT5 was required for TSLP-dependent DC activation, including upregulation of the expression of costimulatory molecules and chemokine production. Furthermore, TH2 responses in mice with DC-specific loss of STAT5 resembled those seen in mice deficient in the receptor for TSLP. Our results show that the TSLP-STAT5 axis in DCs is a critical component for the promotion of type 2 immunity at barrier surfaces.
Figures



References
-
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. - PubMed
-
- McKenna HJ, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. - PubMed
-
- Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

