High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities
- PMID: 23437148
- PMCID: PMC3577892
- DOI: 10.1371/journal.pone.0056506
High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities
Abstract
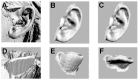
Introduction: Autologous techniques for the reconstruction of pediatric microtia often result in suboptimal aesthetic outcomes and morbidity at the costal cartilage donor site. We therefore sought to combine digital photogrammetry with CAD/CAM techniques to develop collagen type I hydrogel scaffolds and their respective molds that would precisely mimic the normal anatomy of the patient-specific external ear as well as recapitulate the complex biomechanical properties of native auricular elastic cartilage while avoiding the morbidity of traditional autologous reconstructions.
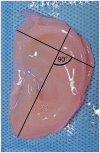
Methods: Three-dimensional structures of normal pediatric ears were digitized and converted to virtual solids for mold design. Image-based synthetic reconstructions of these ears were fabricated from collagen type I hydrogels. Half were seeded with bovine auricular chondrocytes. Cellular and acellular constructs were implanted subcutaneously in the dorsa of nude rats and harvested after 1 and 3 months.
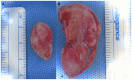
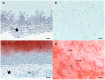
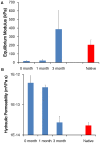
Results: Gross inspection revealed that acellular implants had significantly decreased in size by 1 month. Cellular constructs retained their contour/projection from the animals' dorsa, even after 3 months. Post-harvest weight of cellular constructs was significantly greater than that of acellular constructs after 1 and 3 months. Safranin O-staining revealed that cellular constructs demonstrated evidence of a self-assembled perichondrial layer and copious neocartilage deposition. Verhoeff staining of 1 month cellular constructs revealed de novo elastic cartilage deposition, which was even more extensive and robust after 3 months. The equilibrium modulus and hydraulic permeability of cellular constructs were not significantly different from native bovine auricular cartilage after 3 months.
Conclusions: We have developed high-fidelity, biocompatible, patient-specific tissue-engineered constructs for auricular reconstruction which largely mimic the native auricle both biomechanically and histologically, even after an extended period of implantation. This strategy holds immense potential for durable patient-specific tissue-engineered anatomically proper auricular reconstructions in the future.
Conflict of interest statement
Figures



References
-
- Shieh SJ, Terada S, Vacanti JP (2004) Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials 25: 1545–1557. - PubMed
-
- Bichara DA, O'Sullivan NA, Pomerantseva I, Zhao X, Sundback CA, et al. (2012) The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev 18: 51–61. - PubMed
-
- Haisch A, Klaring S, Groger A, Gebert C, Sittinger M (2002) A tissue-engineering model for the manufacture of auricular-shaped cartilage implants. Eur Arch Otorhinolaryngol 259: 316–321. - PubMed
-
- Rodriguez A, Cao YL, Ibarra C, Pap S, Vacanti M, et al. (1999) Characteristics of cartilage engineered from human pediatric auricular cartilage. Plast Reconstr Surg 103: 1111–1119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

