Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response
- PMID: 23437178
- PMCID: PMC3577871
- DOI: 10.1371/journal.pone.0056586
Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response
Erratum in
- PLoS One. 2013;8(6). doi:10.1371/annotation/dd60f248-ca3f-4824-a3b4-c07aefb1c7c3. Adzemovic, Milena Z [corrected to Adzemovic, Milena V]
-
Correction: imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response.PLoS One. 2015 Apr 7;10(4):e0123142. doi: 10.1371/journal.pone.0123142. eCollection 2015. PLoS One. 2015. PMID: 25849593 Free PMC article. No abstract available.
Abstract
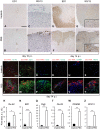
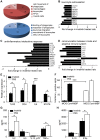
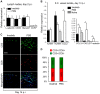
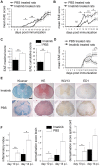
Central nervous system (CNS) disorders such as ischemic stroke, multiple sclerosis (MS) or Alzheimers disease are characterized by the loss of blood-brain barrier (BBB) integrity. Here we demonstrate that the small tyrosine kinase inhibitor imatinib enhances BBB integrity in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis (MS). Treatment was accompanied by decreased CNS inflammation and demyelination and especially reduced T-cell recruitment. This was supported by downregulation of the chemokine receptor (CCR) 2 in CNS and lymph nodes, and by modulation of the peripheral immune response towards an anti-inflammatory phenotype. Interestingly, imatinib ameliorated neuroinflammation, even when the treatment was initiated after the clinical manifestation of the disease. We have previously shown that imatinib reduces BBB disruption and stroke volume after experimentally induced ischemic stroke by targeting platelet-derived growth factor receptor -α (PDGFR-α) signaling. Here we demonstrate that PDGFR-α signaling is a central regulator of BBB integrity during neuroinflammation and therefore imatinib should be considered as a potentially effective treatment for MS.
Conflict of interest statement
Figures


References
-
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD (2006) Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1: 223–236. - PubMed
-
- Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12: 623–635. - PubMed
-
- Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23: 683–747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

