Ultrasound-mediated stimulation of microbubbles after acute myocardial infarction and reperfusion ameliorates left-ventricular remodelling in mice via improvement of borderzone vascularization
- PMID: 23437254
- PMCID: PMC3577680
- DOI: 10.1371/journal.pone.0056841
Ultrasound-mediated stimulation of microbubbles after acute myocardial infarction and reperfusion ameliorates left-ventricular remodelling in mice via improvement of borderzone vascularization
Abstract
Aims: Post-infarction remodelling (PIR) determines left-ventricular (LV) function and prognosis after myocardial infarction. The aim of this study was to evaluate transthoracic ultrasound-mediated microbubble stimulation (UMS) as a novel gene- and cell-free therapeutic option after acute myocardial infarction and reperfusion (AMI/R) in mice.
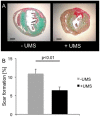
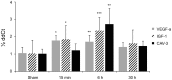
Methods and results: For myocardial delivery of UMS, a novel therapeutic ultrasound-system (TIPS, Philips Medical) and commercially available microbubbles (BR1, Bracco Suisse SA) were utilized in a closed-chest mouse model. UMS was performed as myocardial post-conditioning (PC) on day four after 30 minutes of coronary occlusion and reperfusion. LV-morphology, as well as global and regional function were measured repeatedly with reconstructive 3-dimensional echocardiography applying an additional low-dose dobutamine protocol after two weeks. Scar size was quantified by means of histomorphometry. A total of 41 mice were investigated; 17 received PC with UMS. Mean ejection fraction (EF) prior UMS was similar in both groups 53%±10 (w/o UMS) and 53%±14 (UMS, p = 0.5), reflecting comparable myocardial mass at risk 17%±8 (w/o UMS), 16%±13 (UMS, p = 0.5). Two weeks after AMI/R, mice undergoing UMS demonstrated significantly better global LV-function (EF = 53%±7) as compared to the group without PC (EF = 39%±11, p<0.01). The fraction of akinetic myocardial mass was significantly lower among mice undergoing UMS after AMI/R [27%±10 (w/o UMS), 13%±8 (UMS), p<0.001)]. Our experiments showed a fast onset of transient, UMS-induced upregulation of vascular-endothelial and insulin-like growth factor (VEGF-a, IGF-1), as well as caveolin-3 (Cav-3). The mice undergoing PC with UMS after AMI/R showed a significantly lower scar size. In addition, the microvascular density was significantly higher in the borderzone of UMS-treated animals.
Conclusion: UMS following AMI/R ameliorates PIR in mice via up-regulation of VEGF-a, IGF-1 and Cav-3, and consecutive improvement of myocardial borderzone vascularization.
Conflict of interest statement
Figures





References
-
- Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172. - PubMed
-
- Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101: 2981–2988. - PubMed
-
- Gaudron P, Eilles C, Kugler I, Ertl G (1993) Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation 87: 755–763. - PubMed
-
- Cittadini A, Monti MG, Petrillo V, Esposito G, Imparato G, et al. (2011) Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail 13: 1264–1274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

