Improved BM212 MmpL3 inhibitor analogue shows efficacy in acute murine model of tuberculosis infection
- PMID: 23437287
- PMCID: PMC3578785
- DOI: 10.1371/journal.pone.0056980
Improved BM212 MmpL3 inhibitor analogue shows efficacy in acute murine model of tuberculosis infection
Abstract
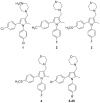
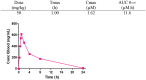
1,5-Diphenyl pyrroles were previously identified as a class of compounds endowed with high in vitro efficacy against M. tuberculosis. To improve the physical chemical properties and drug-like parameters of this class of compounds, a medicinal chemistry effort was undertaken. By selecting the optimal substitution patterns for the phenyl rings at N1 and C5 and by replacing the thiomorpholine moiety with a morpholine one, a new series of compounds was produced. The replacement of the sulfur with oxygen gave compounds with lower lipophilicity and improved in vitro microsomal stability. Moreover, since the parent compound of this family has been shown to target MmpL3, mycobacterial mutants resistant to two compounds have been isolated and characterized by sequencing the mmpL3 gene; all the mutants showed point mutations in this gene. The best compound identified to date was progressed to dose-response studies in an acute murine TB infection model. The resulting ED(99) of 49 mg/Kg is within the range of commonly employed tuberculosis drugs, demonstrating the potential of this chemical series. The in vitro and in vivo target validation evidence presented here adds further weight to MmpL3 as a druggable target of interest for anti-tubercular drug discovery.
Conflict of interest statement
Figures

References
-
- Global Tuberculosis Control: WHO report 2011, World Health Organization, Geneva (WHO/HTM/TB/2011.16).
-
- Treatment of Tuberculosis: guidelines for national programmes 4th ed., World Health Organization, Geneva (WHO/HTM/TB/2009.420).
-
- Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response (2010) World Health Organization, Geneva (WHO/HTM/TB/2010.3).
-
- Biava M, Fioravanti R, Porretta GC, Sleiter G, Ettorre A, et al. (1997) New toluidine derivatives with antimycobacterial and antifungal activities. Med Chem Res 7: 228–250.
-
- Fioravanti R, Biava M, Donnarumma S, Porretta GC, Simonetti M, et al. (1996) Synthesis and microbiological evaluation of (N-heteroaryl)arylmethanamines and their Shiff bases. II Farmaco 51: 643–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

