An extract of Artemisia dracunculus L. inhibits ubiquitin-proteasome activity and preserves skeletal muscle mass in a murine model of diabetes
- PMID: 23437325
- PMCID: PMC3577785
- DOI: 10.1371/journal.pone.0057112
An extract of Artemisia dracunculus L. inhibits ubiquitin-proteasome activity and preserves skeletal muscle mass in a murine model of diabetes
Abstract
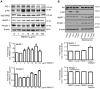
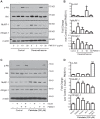
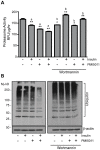
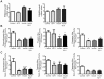
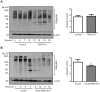
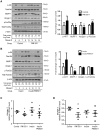
Impaired insulin signaling is a key feature of type 2 diabetes and is associated with increased ubiquitin-proteasome-dependent protein degradation in skeletal muscle. An extract of Artemisia dracunculus L. (termed PMI5011) improves insulin action by increasing insulin signaling in skeletal muscle. We sought to determine if the effect of PMI5011 on insulin signaling extends to regulation of the ubiquitin-proteasome system. C2C12 myotubes and the KK-A(y) murine model of type 2 diabetes were used to evaluate the effect of PMI5011 on steady-state levels of ubiquitylation, proteasome activity and expression of Atrogin-1 and MuRF-1, muscle-specific ubiquitin ligases that are upregulated with impaired insulin signaling. Our results show that PMI5011 inhibits proteasome activity and steady-state ubiquitylation levels in vitro and in vivo. The effect of PMI5011 is mediated by PI3K/Akt signaling and correlates with decreased expression of Atrogin-1 and MuRF-1. Under in vitro conditions of hormonal or fatty acid-induced insulin resistance, PMI5011 improves insulin signaling and reduces Atrogin-1 and MuRF-1 protein levels. In the KK-A(y) murine model of type 2 diabetes, skeletal muscle ubiquitylation and proteasome activity is inhibited and Atrogin-1 and MuRF-1 expression is decreased by PMI5011. PMI5011-mediated changes in the ubiquitin-proteasome system in vivo correlate with increased phosphorylation of Akt and FoxO3a and increased myofiber size. The changes in Atrogin-1 and MuRF-1 expression, ubiquitin-proteasome activity and myofiber size modulated by PMI5011 in the presence of insulin resistance indicate the botanical extract PMI5011 may have therapeutic potential in the preservation of muscle mass in type 2 diabetes.
Conflict of interest statement
Figures


References
-
- Bassel-Duby R, Olson EN (2006) Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37. - PubMed
-
- Wang X, Hu Z, Hu J, Du J, Mitch WE (2006) Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168. - PubMed
-
- Mitch WE, Goldberg AL (1996) Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

