Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents
- PMID: 23438748
- PMCID: PMC3583372
- DOI: 10.1016/j.chembiol.2012.11.010
Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents
Abstract
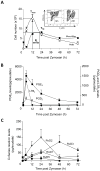
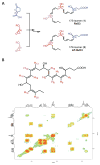
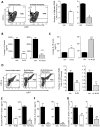
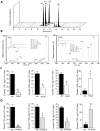
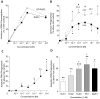
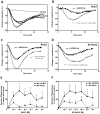
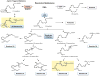
Resolvins are a family of n-3 lipid mediators initially identified in resolving inflammatory exudates that temper inflammatory responses to promote catabasis. Here, temporal metabololipidomics with self-limited resolving exudates revealed that resolvin (Rv) D3 has a distinct time frame from other lipid mediators, appearing late in the resolution phase. Using synthetic materials prepared by stereocontrolled total organic synthesis and metabololipidomics, we established complete stereochemistry of RvD3 and its aspirin-triggered 17R-epimer (AT-RvD3). Both synthetic resolvins potently regulated neutrophils and mediators, reducing murine peritonitis and dermal inflammation. RvD3 and AT-RvD3 displayed leukocyte-directed actions, e.g., blocking human neutrophil transmigration and enhancing macrophage phagocytosis and efferocytosis. These results position RvD3 uniquely within the inflammation-resolution time frame to vantage and contribute to the beneficial actions of aspirin and essential n-3 fatty acids.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Resolving resolvins.Chem Biol. 2013 Feb 21;20(2):138-40. doi: 10.1016/j.chembiol.2013.02.003. Chem Biol. 2013. PMID: 23438740
References
-
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. - PubMed
-
- Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. - PubMed
-
- Brady HR, Serhan CN. Adhesion promotes transcellular leukotriene biosynthesis during neutrophil-glomerular endothelial cell interactions: inhibition by antibodies against CD18 and L-selectin. Biochem Biophys Res Commun. 1992;186:1307–1314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

