O-GlcNAcylation and 5-methylcytosine oxidation: an unexpected association between OGT and TETs
- PMID: 23438858
- PMCID: PMC3770526
- DOI: 10.1016/j.molcel.2013.02.006
O-GlcNAcylation and 5-methylcytosine oxidation: an unexpected association between OGT and TETs
Abstract
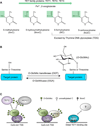
Three recent studies, including one in this issue of Molecular Cell, document unexpected physical and functional interactions between two unrelated enzymes: OGT, which transfers O-GlcNAc to serine/threonine residues of numerous cellular proteins, and TET-family dioxygenases, which successively oxidize 5-methylcytosine in DNA.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Comment on
-
TET2 promotes histone O-GlcNAcylation during gene transcription.Nature. 2013 Jan 24;493(7433):561-4. doi: 10.1038/nature11742. Epub 2012 Dec 9. Nature. 2013. PMID: 23222540 Free PMC article.
-
Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells.Mol Cell. 2013 Feb 21;49(4):645-56. doi: 10.1016/j.molcel.2012.12.019. Epub 2013 Jan 24. Mol Cell. 2013. PMID: 23352454
-
TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS.EMBO J. 2013 Mar 6;32(5):645-55. doi: 10.1038/emboj.2012.357. Epub 2013 Jan 25. EMBO J. 2013. PMID: 23353889 Free PMC article.
References
-
- Chen Q, Chen Y, Bian C, Fujiki R, Yu X. Nature. 2013;493:561–564. Published online December 9, 2012. http://dx.doi.org/10.1038/nature11742. - DOI - PMC - PubMed
-
- Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, Dawson MA, Volkmar M, Putmans P, Calonne E, et al. EMBO J. 2013 in press. Published online January 25, 2013. http://dx.doi.org/10.1038/emboj.2012.357. - DOI - PMC - PubMed
-
- Gambetta MC, Oktaba K, Müller J. Science. 2009;325:93–96. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

