Super-resolution scanning patch clamp reveals clustering of functional ion channels in adult ventricular myocyte
- PMID: 23438901
- PMCID: PMC3899650
- DOI: 10.1161/CIRCRESAHA.111.300445
Super-resolution scanning patch clamp reveals clustering of functional ion channels in adult ventricular myocyte
Abstract
Rationale: Compartmentation of ion channels on the cardiomyocyte surface is important for electric propagation and electromechanical coupling. The specialized T-tubule and costameric structures facilitate spatial coupling of various ion channels and receptors. Existing methods such as immunofluorescence and patch clamp techniques are limited in their ability to localize functional ion channels. As such, a correlation between channel protein location and channel function remains incomplete.
Objective: To validate a method that permits routine imaging of the topography of a live cardiomyocyte and study clustering of functional ion channels from a specific microdomain.
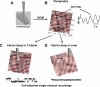
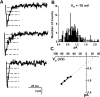
Methods and results: We used scanning ion conductance microscopy and conventional cell-attached patch clamp with a software modification that allows controlled increase of pipette tip diameter. The sharp nanopipette used for topography scan was modified into a larger patch pipette that could be positioned with nanoscale precision to a specific site of interest (crest, groove, or T-tubules of cardiomyocytes) and sealed to the membrane for cell-attached recording of ion channels. Using this method, we significantly increased the probability of detecting activity of L-type calcium channels in the T-tubules of ventricular cardiomyocytes. We also demonstrated that active sodium channels do not distribute homogenously on the sarcolemma instead, they segregate into clusters of various densities, most crowded in the crest region, that are surrounded by areas virtually free of functional sodium channels.
Conclusions: Our new method substantially increases the throughput of recording location-specific functional ion channels on the cardiomyocyte sarcolemma, thereby allowing characterization of ion channels in relation to the microdomain where they reside.
Figures



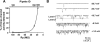

Comment in
-
High-resolution scanning patch clamp: life on the nanosurface.Circ Res. 2013 Apr 12;112(8):1088-90. doi: 10.1161/CIRCRESAHA.113.301212. Circ Res. 2013. PMID: 23580769 Free PMC article. No abstract available.
References
-
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the camp signaling pathway. J Biol Chem. 2000;275:41447–41457. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: Alterations during cardiac remodelling. Cardiovasc Res. 2008;77:315–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL106632/HL/NHLBI NIH HHS/United States
- BB/D018595/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- RG/12/18/30088/BHF_/British Heart Foundation/United Kingdom
- C19021/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 GM057691/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

