A cholinergic mechanism for reward timing within primary visual cortex
- PMID: 23439124
- PMCID: PMC3597441
- DOI: 10.1016/j.neuron.2012.12.039
A cholinergic mechanism for reward timing within primary visual cortex
Abstract
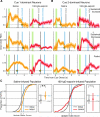
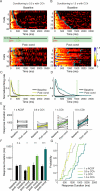
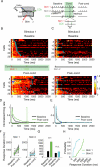
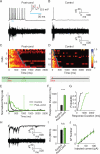
Neurons in rodent primary visual cortex (V1) relate operantly conditioned stimulus-reward intervals with modulated patterns of spiking output, but little is known about the locus or mechanism of this plasticity. Here we show that cholinergic basal forebrain projections to V1 are necessary for the neural acquisition, but not the expression, of reward timing in the visual cortex of awake, behaving animals. We then mimic reward timing in vitro by pairing white matter stimulation with muscarinic receptor activation at a fixed interval and show that this protocol results in the prolongation of electrically evoked spike train durations out to the conditioned interval. Together, these data suggest that V1 possesses the circuitry and plasticity to support reward time prediction learning and the cholinergic system serves as an important reinforcement signal which, in vivo, conveys to the cortex the outcome of behavior.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. - PubMed
-
- Bekisz M, Garkun Y, Wabno J, Hess G, Wrobel A, Kossut M. Increased excitability of cortical neurons induced by associative learning: an ex vivo study. The European journal of neuroscience. 2010;32:1715–1725. - PubMed
-
- Brocher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

