Performance variability of seven commonly used self-monitoring of blood glucose systems: clinical considerations for patients and providers
- PMID: 23439170
- PMCID: PMC3692226
- DOI: 10.1177/193229681300700117
Performance variability of seven commonly used self-monitoring of blood glucose systems: clinical considerations for patients and providers
Abstract
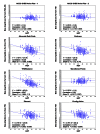
Background: Blood glucose data are frequently used in clinical decision making, thus it is critical that self-monitoring of blood glucose (SMBG) systems consistently provide accurate results. Concerns about SMBG accuracy have prompted the development of newly proposed International Organization for Standardization (ISO) standards: ≥ 95% of individual glucose results shall fall within ± 15 mg/dl of the results of the manufacturer's reference procedure at glucose concentrations <100 mg/dl and within ± 15% for values ≥ 100 mg/dl. We evaluated seven marketed systems against the current and proposed ISO criteria (criterion A).
Method: Capillary blood samples were collected from 100 subjects and tested on seven systems: Accu-Chek Aviva Plus, Advocate Redi-Code, Element, Embrace, Prodigy Voice, TRUEbalance, and WaveSense Presto. Results were compared with manufacturer's documented reference system, YSI or perchloric acid hexokinase; three different strip lots from each system were tested on each subject, in duplicate.
Results: Compared against current ISO criteria (≥ 95% within ± 15 mg/dl for values <75 mg/dl and ± 20% for values ≥ 75 mg/dl) the Accu-Chek Aviva Plus, Element, and WaveSense Presto systems met accuracy criteria. However, only the Accu-Chek Aviva Plus met the proposed ISO criteria (criterion A) in all three lots. The other six systems failed to meet the criteria in at least two of the three lots, showing lot-to-lot variability, high/low bias, and variations due to hematocrit.
Conclusions: Inaccurate SMBG readings can potentially adversely impact clinical decision making and outcomes. Clinicians can reduce controllable variables by prescribing accurate SMBG systems. Adherence to the proposed ISO criteria should enhance patient safety by improving the accuracy of SMBG systems.
© 2013 Diabetes Technology Society.
Figures
Comment in
-
Variability of blood glucose meters for patient self-testing: analysis of the article by Brazg and coauthors.J Diabetes Sci Technol. 2013 Jan 1;7(1):153-5. doi: 10.1177/193229681300700118. J Diabetes Sci Technol. 2013. PMID: 23439171 Free PMC article.
References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. - PubMed
-
- Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: Results from the structured testing program study. Diabetes Care. 2011;34(2):262–7. - PMC - PubMed
-
- Bonomo K, De Salve A, Fiora E, Mularoni E, Massucco P, Poy P, Pomero A, Cavalot F, Anfossi G, Trovati M. Evaluation of a simple policy for pre- and post-prandial blood glucose self-monitoring in people with type 2 diabetes not on insulin. Diabetes Res Clin Pract. 2010;87(2):246–51. - PubMed
-
- Kempf K, Kruse J, Martin S. Rosso-in-praxi: A self-monitoring of blood glucose-structured 12-week lifestyle intervention significantly improves glucometabolic control of patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2010;12(7):547–53. - PubMed
-
- International Organization for Standardization. ISO 15197:2003. In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous