The SRC family tyrosine kinase HCK and the ETS family transcription factors SPIB and EHF regulate transcytosis across a human follicle-associated epithelium model
- PMID: 23439650
- PMCID: PMC3624422
- DOI: 10.1074/jbc.M112.437475
The SRC family tyrosine kinase HCK and the ETS family transcription factors SPIB and EHF regulate transcytosis across a human follicle-associated epithelium model
Abstract
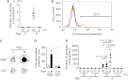
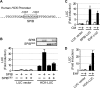
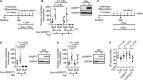
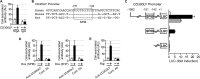
A critical step in the induction of adaptive mucosal immunity is antigen transcytosis, in which luminal antigens are transported to organized lymphoid tissues across the follicle-associated epithelium (FAE) of Peyer's patches. However, virtually nothing is known about intracellular signaling proteins and transcription factors that regulate apical-to-basolateral transcytosis. The FAE can transcytose a variety of luminal contents, including inert particles, in the absence of specific opsonins. Furthermore, it expresses receptors for secretory immunoglobulin A (SIgA), the main antibody in mucosal secretions, and uses them to efficiently transcytose SIgA-opsonized particles present in the lumen. Using a human FAE model, we show that the tyrosine kinase HCK regulates apical-to-basolateral transcytosis of non-opsonized and SIgA-opsonized particles. We also show that, in cultured intestinal epithelial cells, ectopic expression of the transcription factor SPIB or EHF is sufficient to activate HCK-dependent apical-to-basolateral transcytosis of these particles. Our results provide the first molecular insights into the intracellular regulation of antigen sampling at mucosal surfaces.
Figures




References
-
- Kraehenbuhl J.-P., Neutra M. R. (2000) Epithelial M cells. Differentiation and function. Annu. Rev. Cell Dev. Biol. 16, 301–332 - PubMed
-
- Neutra M. R., Kozlowski P. A. (2006) Mucosal vaccines. The promise and the challenge. Nat. Rev. Immunol. 6, 148–158 - PubMed
-
- Brandtzaeg P. (2010) Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39, 303–355 - PubMed
-
- Kunisawa J., Kurashima Y., Kiyono H. (2012) Gut-associated lymphoid tissues for the development of oral vaccines. Adv. Drug. Deliv. Rev. 64, 523–530 - PubMed
-
- Schulz O., Pabst O. (2013) Antigen sampling in the small intestine. Trends Immunol., in press - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

