Investigating the role of viral integral membrane proteins in promoting the assembly of nepovirus and comovirus replication factories
- PMID: 23439982
- PMCID: PMC3557413
- DOI: 10.3389/fpls.2012.00313
Investigating the role of viral integral membrane proteins in promoting the assembly of nepovirus and comovirus replication factories
Abstract
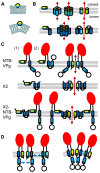
Formation of plant virus membrane-associated replication factories requires the association of viral replication proteins and viral RNA with intracellular membranes, the recruitment of host factors and the modification of membranes to form novel structures that house the replication complex. Many viruses encode integral membrane proteins that act as anchors for the replication complex. These hydrophobic proteins contain transmembrane domains and/or amphipathic helices that associate with the membrane and modify its structure. The comovirus Co-Pro and NTP-binding (NTB, putative helicase) proteins and the cognate nepovirus X2 and NTB proteins are among the best characterized plant virus integral membrane replication proteins and are functionally related to the picornavirus 2B, 2C, and 3A membrane proteins. The identification of membrane association domains and analysis of the membrane topology of these proteins is discussed. The evidence suggesting that these proteins have the ability to induce membrane proliferation, alter the structure and integrity of intracellular membranes, and modulate the induction of symptoms in infected plants is also reviewed. Finally, areas of research that need further investigation are highlighted.
Keywords: integral membrane proteins; intracellular membranes; membrane remodeling; picornavirales; plant–virus interactions; protein–membrane interactions; secoviridae; viral replication complexes.
Figures

References
-
- Agirre A., Barco A., Carrasco L., Nieva J. L. (2002). Viroporin-mediated membrane permeabilization. Pore formation by nonstructural poliovirus 2B protein. J. Biol. Chem. 277 40434–40441 - PubMed
-
- Bienz K., Egger D., Pfister T. (1994). Characteristics of the poliovirus replication complex. Arch. Virol. Suppl. 9 147–157 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

