Protective Effects of Oleic Acid Against Palmitic Acid-Induced Apoptosis in Pancreatic AR42J Cells and Its Mechanisms
- PMID: 23440052
- PMCID: PMC3579104
- DOI: 10.4196/kjpp.2013.17.1.43
Protective Effects of Oleic Acid Against Palmitic Acid-Induced Apoptosis in Pancreatic AR42J Cells and Its Mechanisms
Abstract
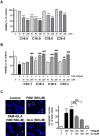
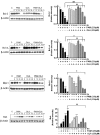
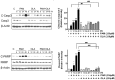
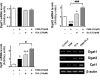
Palmitic acid (PAM), one of the most common saturated fatty acid (SFA) in animals and plants, has been shown to induce apoptosis in exocrine pancreatic AR42J cells. In this study, we investigated cellular mechanisms underlying protective effects of oleic acid (OLA) against the lipotoxic actions of PAM in AR42J cells. Exposure of cells to long-chain SFA induced apoptotic cell death determined by MTT cell viability assay and Hoechst staining. Co-treatment of OLA with PAM markedly protected cells against PAM-induced apoptosis. OLA significantly attenuated the PAM-induced increase in the levels of pro-apoptotic Bak protein, cleaved forms of apoptotic proteins (caspase-3, PARP). On the contrary, OLA restored the decreased levels of anti-apoptotic Bcl-2 family proteins (Bcl-2, Bcl-xL, and Mcl-1) in PAM-treated cells. OLA also induced up-regulation of the mRNA expression of Dgat2 and Cpt1 genes which are involved in triacylglycerol (TAG) synthesis and mitochondrial β-oxidation, respectively. Intracellular TAG accumulation was increased by OLA supplementation in accordance with enhanced expression of Dgat2 gene. These results indicate that restoration of anti-apoptotic/pro-apoptotic protein balance from apoptosis toward cell survival is involved in the cytoprotective effects of OLA against PAM-induced apoptosis in pancreatic AR42J cells. In addition, OLA-induced increase in TAG accumulation and up-regulation of Dgat2 and Cpt1 gene expressions may be possibly associated in part with the ability of OLA to protect cells from deleterious actions of PAM.
Keywords: Anti-apoptotic/pro-apoptotic proteins; Apoptosis; Oleic acid; Palmitic acid; Pancreatic AR42J cells.
Figures

Similar articles
-
Palmitate induced lipoapoptosis of exocrine pancreas AR42J cells.Apoptosis. 2006 May;11(5):717-24. doi: 10.1007/s10495-006-5425-3. Apoptosis. 2006. PMID: 16532273
-
Fatty Acids Have Different Adipogenic Differentiation Potentials in Stromal Vascular Cells Isolated from Abdominal Fat in Laying Hens.Lipids. 2017 Jun;52(6):513-522. doi: 10.1007/s11745-017-4261-2. Epub 2017 May 18. Lipids. 2017. PMID: 28523479
-
Palmitic acid and oleic acid differentially regulate choline transporter-like 1 levels and glycerolipid metabolism in skeletal muscle cells.Lipids. 2014 Aug;49(8):731-44. doi: 10.1007/s11745-014-3925-4. Epub 2014 Jun 28. Lipids. 2014. PMID: 24972900
-
PAM, OLA, and LNA are Differentially Taken Up and Trafficked Via Different Metabolic Pathways in Porcine Adipocytes.Lipids. 2017 Nov;52(11):929-938. doi: 10.1007/s11745-017-4302-x. Epub 2017 Oct 20. Lipids. 2017. PMID: 29058170
-
M. leprae inhibits apoptosis in THP-1 cells by downregulation of Bad and Bak and upregulation of Mcl-1 gene expression.BMC Microbiol. 2006 Sep 18;6:78. doi: 10.1186/1471-2180-6-78. BMC Microbiol. 2006. PMID: 16978419 Free PMC article.
Cited by
-
Shigella flexneri remodeling and consumption of host lipids during infection.J Bacteriol. 2023 Dec 19;205(12):e0032023. doi: 10.1128/jb.00320-23. Epub 2023 Nov 22. J Bacteriol. 2023. PMID: 37991380 Free PMC article.
-
The Distinct Effects of Palmitic and Oleic Acid on Pancreatic Beta Cell Function: The Elucidation of Associated Mechanisms and Effector Molecules.Front Pharmacol. 2019 Jan 21;9:1554. doi: 10.3389/fphar.2018.01554. eCollection 2018. Front Pharmacol. 2019. PMID: 30719005 Free PMC article.
-
Molecular Mechanisms of Apoptosis Induction and Its Regulation by Fatty Acids in Pancreatic β-Cells.Int J Mol Sci. 2021 Apr 20;22(8):4285. doi: 10.3390/ijms22084285. Int J Mol Sci. 2021. PMID: 33924206 Free PMC article. Review.
-
Free fatty acids support oligodendrocyte survival in a mouse model of amyotrophic lateral sclerosis.Front Cell Neurosci. 2023 May 12;17:1081190. doi: 10.3389/fncel.2023.1081190. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37252191 Free PMC article.
-
Chemical Composition and Antiproliferative Effects of a Methanol Extract of Aspongopus chinensis Dallas.Evid Based Complement Alternat Med. 2019 May 30;2019:2607086. doi: 10.1155/2019/2607086. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31275405 Free PMC article.
References
-
- Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9:1–10. - PubMed
-
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. - PubMed
-
- Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. - PubMed
-
- Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

