Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits
- PMID: 23440208
- PMCID: PMC3600471
- DOI: 10.1073/pnas.1216867110
Genotype-phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of K(v)7.2 potassium channel subunits
Abstract
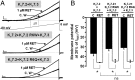
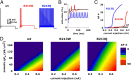
Mutations in the K(V)7.2 gene encoding for voltage-dependent K(+) channel subunits cause neonatal epilepsies with wide phenotypic heterogeneity. Two mutations affecting the same positively charged residue in the S4 domain of K(V)7.2 have been found in children affected with benign familial neonatal seizures (R213W mutation) or with neonatal epileptic encephalopathy with severe pharmacoresistant seizures and neurocognitive delay, suppression-burst pattern at EEG, and distinct neuroradiological features (R213Q mutation). To examine the molecular basis for this strikingly different phenotype, we studied the functional characteristics of mutant channels by using electrophysiological techniques, computational modeling, and homology modeling. Functional studies revealed that, in homomeric or heteromeric configuration with K(V)7.2 and/or K(V)7.3 subunits, both mutations markedly destabilized the open state, causing a dramatic decrease in channel voltage sensitivity. These functional changes were (i) more pronounced for channels incorporating R213Q- than R213W-carrying K(V)7.2 subunits; (ii) proportional to the number of mutant subunits incorporated; and (iii) fully restored by the neuronal K(v)7 activator retigabine. Homology modeling confirmed a critical role for the R213 residue in stabilizing the activated voltage sensor configuration. Modeling experiments in CA1 hippocampal pyramidal cells revealed that both mutations increased cell firing frequency, with the R213Q mutation prompting more dramatic functional changes compared with the R213W mutation. These results suggest that the clinical disease severity may be related to the extent of the mutation-induced functional K(+) channel impairment, and set the preclinical basis for the potential use of K(v)7 openers as a targeted anticonvulsant therapy to improve developmental outcome in neonates with K(V)7.2 encephalopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang HS, et al. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science. 1998;282(5395):1890–1893. - PubMed
-
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283(5748):673–676. - PubMed
-
- Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: Molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 2011;26(5):365–376. - PubMed
-
- Biervert C, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279(5349):403–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

