Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the β2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: a randomised trial
- PMID: 23440247
- PMCID: PMC3664377
- DOI: 10.1136/thoraxjnl-2012-202606
Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the β2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: a randomised trial
Abstract
Background: The inhaled corticosteroid fluticasone furoate (FF) in combination with the long-acting β2 agonist vilanterol (VI) is in development for asthma and chronic obstructive pulmonary disease.
Objective: To assess the safety and tolerability of FF/VI over 52 weeks in patients with asthma.
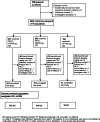
Methods: Patients (aged ≥12 years; on inhaled corticosteroid) were randomised (2:2:1) to FF/VI 100/25 µg or FF/VI 200/25 µg once daily in the evening, or fluticasone propionate (FP) 500 µg twice daily. Safety evaluations included adverse events (AEs), non-fasting glucose, potassium, 24-h urinary cortisol excretion, ophthalmic assessments, heart rate and pulse rate.
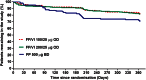
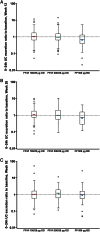
Results: On-treatment AEs were similar across groups (FF/VI 66-69%; 73% FP). Oral candidiasis/oropharyngeal candidiasis was more common with FF/VI (6-7%) than FP (3%). Twelve serious AEs were reported; one (worsening hepatitis B on FP) was considered drug related. Statistically significant cortisol suppression was seen with FP compared with both FF/VI groups at Weeks 12 and 28 (ratios [95% CI] to FP ranged from 1.43 [1.11 to 1.84] to 1.67 [1.34 to 2.08]; p≤0.006), but not at Week 52 (ratios to FP were 1.05 [0.83 to 1.33] for FF/VI 100/25 µg and 1.09 [0.87 to 1.38] for FF/VI 200/25 µg). No clinically important changes in non-fasting glucose, potassium, QT interval corrected using Fridericia's formula (QTc[F]) or ophthalmic assessments were reported. Pulse rate (10 min post dose [Tmax], Week 52) was significantly increased with FF/VI versus FP (3.4 bpm, 95% CI 1.3 to 5.6; p=0.002 [FF/VI 100/25 µg]; 3.4 bpm, 95% CI 1.2 to 5.6; p=0.003 [FF/VI 200/25 µg]). Mean heart rate (24-h Holter monitoring) decreased from screening values in all groups (0.2-1.1 bpm FF/VI vs 5 bpm FP; Week 52).
Conclusions: FF/VI (100/25 µg or 200/25 µg) administered once daily over 52 weeks was well tolerated by patients aged ≥12 years with asthma. The overall safety profile of FF/VI did not reveal any findings of significant clinical concern. CLINICALTRIALS.GOV: NCT01018186.
Keywords: Asthma.
Figures
Comment in
-
Systemic safety of fluticasone furoate/vilanterol combination.Thorax. 2013 Dec;68(12):1165. doi: 10.1136/thoraxjnl-2013-203910. Epub 2013 Jul 2. Thorax. 2013. PMID: 23821392 No abstract available.
-
Authors' reply to 'Safety and tolerability of fluticasone furoate/vilanterol'.Thorax. 2013 Dec;68(12):1165-6. doi: 10.1136/thoraxjnl-2013-203992. Epub 2013 Jul 2. Thorax. 2013. PMID: 24204004 No abstract available.
References
-
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. Updated 2011. http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=1561 (accessed 16 Jul 2012).
-
- National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) 2007. NHLBI, August; 2007. NIH publication no. 08-4051. http://www.nhlbi.nih.gov/guidelines/asthma/index.htm (accessed 16 Jul 2012).
-
- Colice GL. Emerging therapeutic options for asthma. Am J Manag Care 2011;(Suppl 3):S82–9 - PubMed
-
- Bateman ED, Bleecker ER, Busse W, et al. Dose effect of once-daily fluticasone furoate in persistent asthma: a randomized trial. Resp Med 2012;106:642–50 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical