A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype
- PMID: 23440415
- PMCID: PMC4148560
- DOI: 10.4049/jimmunol.1202441
A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype
Abstract
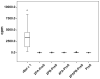
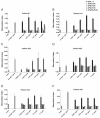
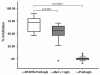
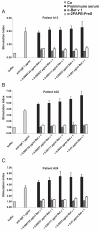
Allergen-specific immunotherapy is the only allergen-specific and disease-modifying treatment for allergy. The construction and characterization of a vaccine for birch pollen allergy is reported. Two nonallergenic peptides, PA and PB, derived from the IgE-reactive areas of the major birch pollen allergen Bet v 1 were fused to the hepatitis B surface protein, PreS, in four recombinant fusion proteins containing different numbers and combinations of the peptides. Fusion proteins expressed in Escherichia coli and purified to homogeneity showed a lack of IgE reactivity and allergenic activity when tested with sera and basophils from patients allergic to birch pollen. Compared to Bet v 1 allergen, peptides PA and PB showed reduced T cell activation in PBMCs from allergic patients, whereas PreS fusion proteins induced less IL-5 and more IL-10 and IFN-γ. Immunization of rabbits with the fusion proteins, in particular with a PreS fusion protein 2PAPB-PreS, containing two copies of each peptide, induced high levels of IgG Abs against the major IgE-reactive site on Bet v 1 and related allergens. These IgG Abs inhibited allergic patients' IgE binding to Bet v 1 better than did IgG induced by immunization with complete Bet v 1. Furthermore, 2PAPB-PreS-induced IgG inhibited Bet v 1-induced basophil activation in allergic patients and CD23-facilitated allergen presentation. Our study exemplifies novel beneficial features for a PreS carrier-based peptide vaccine for birch pollen, which, in addition to the established reduction in allergenic activity, include the enhanced focusing of blocking Ab responses toward IgE epitopes, immunomodulatory activity, and reduction of CD23-facilitated allergen presentation.
Figures




References
-
- Flöistrup H, Swartz J, Bergström A, Alm JS, Scheynius A, van Hage M, Waser M, Braun-Fahrländer C, Schram-Bijkerk D, Huber M, et al. Parsifal Study Group Allergic disease and sensitization in Steiner school children. J. Allergy Clin. Immunol. 2006;117:59–66. - PubMed
-
- Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572–1573.
-
- Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. - PubMed
-
- Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999;341:468–475. - PubMed
-
- Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, Koivikko A, Koller DY, Niggemann B, Norberg LA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J. Allergy Clin. Immunol. 2002;109:251–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

