IRF7-dependent IFN-β production in response to RANKL promotes medullary thymic epithelial cell development
- PMID: 23440417
- PMCID: PMC3608802
- DOI: 10.4049/jimmunol.1203086
IRF7-dependent IFN-β production in response to RANKL promotes medullary thymic epithelial cell development
Abstract
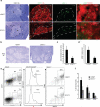
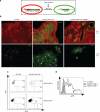
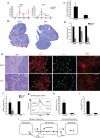
The contributions of IFN regulatory factor (IRF) 3/7 and the type I IFNs IFN-α/β to the innate host defense have been extensively investigated; however, their role in thymic development is less clear. In this study, we show that mice lacking the type I IFN receptor IFN-α/β receptor (IFNAR) or the downstream transcription factor STAT1 harbor a significant reduction in self-Ag-presenting, autoimmune regulator (AIRE)(+) medullary thymic epithelial cells (mTECs). Constitutive IFNAR signaling occurs in the thymic medulla in the absence of infection or inflammation. Receptor activator for NF-κB (RANK) ligand stimulation results in IFN-β upregulation, which in turn inhibits RANK signaling and facilitates AIRE expression in mTECs. Finally, we find that IRF7 is required for thymic IFN-β induction, maintenance of thymic architecture, and mTEC differentiation. We conclude that spatially and temporally coordinated cross talks between the RANK ligand/RANK and IRF7/IFN-β/IFNAR/STAT1 pathways are essential for differentiation of AIRE(+) mTECs.
Figures


References
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
-
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. - PubMed
-
- Finnish-German AC. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. - PubMed
-
- Peterson P, Nagamine K, Scott H, Heino M, Kudoh J, Shimizu N, Antonarakis SE, Krohn KJ. APECED: a monogenic autoimmune disease providing new clues to self-tolerance. Immunol Today. 1998;19:384–386. - PubMed
-
- Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

