KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis
- PMID: 23440420
- PMCID: PMC3736094
- DOI: 10.4049/jimmunol.1202926
KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis
Abstract
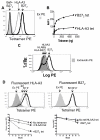
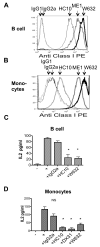
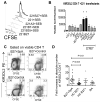
The human leukocyte Ag HLA-B27 (B27) is strongly associated with the spondyloarthritides. B27 can be expressed at the cell surface of APC as both classical β2-microglobulin-associated B27 and B27 free H chain forms (FHC), including disulfide-bonded H chain homodimers (termed B27(2)). B27 FHC forms, but not classical B27, bind to KIR3DL2. HLA-A3, which is not associated with spondyloarthritis (SpA), is also a ligand for KIR3DL2. In this study, we show that B27(2) and B27 FHC bind more strongly to KIR3DL2 than other HLA-class I, including HLA-A3. B27(2) tetramers bound KIR3DL2-transfected cells more strongly than HLA-A3. KIR3DL2Fc bound to HLA-B27-transfected cells more strongly than to cells transfected with other HLA-class I. KIR3DL2Fc pulled down multimeric, dimeric, and monomeric FHC from HLA-B27-expressing cell lines. Binding to B27(2) and B27 FHC stimulated greater KIR3DL2 phosphorylation than HLA-A3. B27(2) and B27 FHC stimulated KIR3DL2CD3ε-transduced T cell IL-2 production to a greater extent than control HLA-class I. KIR3DL2 binding to B27 inhibited NK IFN-γ secretion and promoted greater survival of KIR3DL2(+) CD4 T and NK cells than binding to other HLA-class I. KIR3DL2(+) T cells from B27(+) SpA patients proliferated more in response to Ag presented by syngeneic APC than the same T cell subset from healthy and disease controls. Our results suggest that expansion of KIR3DL2-expressing leukocytes observed in B27(+) SpA may be explained by the stronger interaction of KIR3DL2 with B27 FHC.
Figures



References
-
- Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. HLA-B27 misfolding and spondyloarthropathies. Adv Exp Med Biol. 2009;649:217–234. - PubMed
-
- Fiorillo MT, Sorrentino R. T-cell responses against viral and self-epitopes and HLA-B27 subtypes differentially associated with ankylosing spondylitis. Adv Exp Med Biol. 2009;649:255–262. - PubMed
-
- Lopez de Castro JA. HLA-B27 and the pathogenesis of spondyloarthropathies. Immunol Lett. 2007;108:27–33. - PubMed
-
- Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, Bowness P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–2982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

