An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity
- PMID: 23440422
- PMCID: PMC3919498
- DOI: 10.1158/0008-5472.CAN-13-0382-T
An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity
Abstract
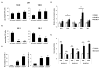
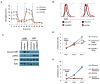
The sterol regulatory element-binding proteins (SREBP) are key transcriptional regulators of lipid metabolism and cellular growth. It has been proposed that SREBP signaling regulates cellular growth through its ability to drive lipid biosynthesis. Unexpectedly, we find that loss of SREBP activity inhibits cancer cell growth and viability by uncoupling fatty acid synthesis from desaturation. Integrated lipid profiling and metabolic flux analysis revealed that cancer cells with attenuated SREBP activity maintain long-chain saturated fatty acid synthesis, while losing fatty acid desaturation capacity. We traced this defect to the uncoupling of fatty acid synthase activity from stearoyl-CoA desaturase 1 (SCD1)-mediated desaturation. This deficiency in desaturation drives an imbalance between the saturated and monounsaturated fatty acid pools resulting in severe lipotoxicity. Importantly, replenishing the monounsaturated fatty acid pool restored growth to SREBP-inhibited cells. These studies highlight the importance of fatty acid desaturation in cancer growth and provide a novel mechanistic explanation for the role of SREBPs in cancer metabolism.
Conflict of interest statement
Dr. Paul Mischel has served as a scientific advisor to Celgene on the mTOR kinase inhibitor program. No other potential conflicts of interest were disclosed.
Figures




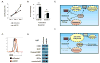
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. - PubMed
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

