Vitamin A and retinoid signaling: genomic and nongenomic effects
- PMID: 23440512
- PMCID: PMC3679380
- DOI: 10.1194/jlr.R030833
Vitamin A and retinoid signaling: genomic and nongenomic effects
Abstract
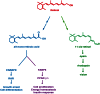
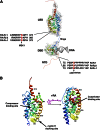
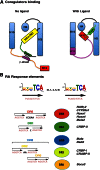
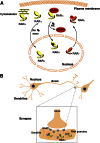
Vitamin A or retinol is arguably the most multifunctional vitamin in the human body, as it is essential from embryogenesis to adulthood. The pleiotropic effects of vitamin A are exerted mainly by one active metabolite, all-trans retinoic acid (atRA), which regulates the expression of a battery of target genes through several families of nuclear receptors (RARs, RXRs, and PPARβ/δ), polymorphic retinoic acid (RA) response elements, and multiple coregulators. It also involves extranuclear and nontranscriptional effects, such as the activation of kinase cascades, which are integrated in the nucleus via the phosphorylation of several actors of RA signaling. However, vitamin A itself proved recently to be active and RARs to be present in the cytosol to regulate translation and cell plasticity. These new concepts expand the scope of the biologic functions of vitamin A and RA.
Keywords: kinase cascade; nuclear receptor; retinoic acid receptor; retinoid X receptor; transcription.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

