Instruments for chorionic villus sampling for prenatal diagnosis
- PMID: 23440775
- PMCID: PMC7050982
- DOI: 10.1002/14651858.CD000114.pub2
Instruments for chorionic villus sampling for prenatal diagnosis
Abstract
Background: Chorionic villus sampling (CVS) is the method of choice for obtaining fetal tissue for prenatal diagnosis before 15 weeks of pregnancy. CVS can be performed using either a transabdominal or transcervical approach. The type of instrument and technique used could have a significant impact on the outcome of the procedure. An ability to manoeuvre the instrument within the uterine cavity without puncturing the gestational sac, to see the tip of the instrument on ultrasound scanning and to minimise the number of instrument passes into the uterus are particularly important.
Objectives: To compare the efficacy and safety of different instruments and techniques used to obtain chorionic tissue in early pregnancy by the transabdominal or transcervical route. Primary outcomes included failure to obtain an adequate sample (greater than 5 mg of chorionic villi), need for reinsertion of the instrument, pain, and miscarriage following the procedure. Secondary outcomes included mean weight of tissue obtained, successful culture, difficult instrument insertion, poor visualisation of instrument, vaginal bleeding following the procedure and cost per procedure.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2012).
Selection criteria: Randomised trials comparing different instruments (forceps, cannula, needle) or techniques for CVS using either transabdominal or transcervical approach.
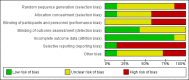
Data collection and analysis: Two review authors assessed eligibility and trial quality.
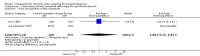
Main results: For transcervical CVS, forceps and cannulae were evaluated in five trials involving 472 women. When a cannula was used, operators failed to obtain an adequate sample (greater than 5 mg of chorionic villi) more often (average risk ratio (RR) 3.81; 95% confidence interval (CI) 1.52 to 9.56). There was no difference in the need for reinsertion of instruments (average RR 2.44; 95% CI 0.83 to 7.20). However, inserting a cannula was more painful (RR 1.93; 95% CI 1.11 to 3.37). There was no difference in spontaneous miscarriage when the use of a cannula was compared with biopsy forceps (RR 1.00; 95% CI 0.14 to 6.96). One study reported the cost of the procedures and found CVS with a cannula to be more expensive (mean difference (MD) $183.7; 95% CI 152.62 to 214.78).When different types of cannulae for transcervical CVS were compared, a Portex cannula was more likely to result in an inadequate sample (RR 2.23; 95% CI 1.25 to 3.98) compared with the silver cannula and to result in a difficult (RR 3.26; 95% CI 1.38 to 7.67) or painful (RR 5.81; 95% CI 1.41 to 23.88) procedure when compared with the aluminium cannula.For transabdominal CVS, two trials comparing different needle techniques were included involving 285 women. One study using an ex vivo system of term placentae was excluded. The included trials compared different continuous negative pressure aspiration techniques with a discontinuous negative pressure system created by a syringe attached to a 20 gauge needle. The studies produced discrepant results. One study found there was no significant difference between groups in the mean weight of chorionic villi obtained (MD 0.40; 95% CI -2.25 to 3.05) or in failure to obtain an adequate sample (more than 5 mg of chorionic villi) on the first attempt (RR 1.02; 95% CI 0.54 to 1.93), whereas the other study found both of these outcomes to be significantly less favourable with the standard discontinuous technique using a syringe (mean weight of chorionic villi obtained: MD -14.80; 95% CI -21.71 to -7.89; failure to obtain an adequate sample on the first attempt: RR 2.73; 95% CI 1.08 to 6.92). There was no difference in rate of miscarriage following the procedure in either study (RR 7.15; 95% CI 0.37 to 136.50; RR 2.93; 95% CI 0.12 to 70.00). Perceived pain by the patient was similar between groups (MD 0.00; 95% CI -0.04 to 0.04) as was success of culture (no failed cases).
Authors' conclusions: For transcervical CVS, although there is some evidence to support the use of small forceps instead of cannulae, the evidence is not strong enough to support change in practice for clinicians who have become familiar with a particular technique. For transabdominal CVS, based on current evidence, there is no difference in clinically important outcomes with the use of a continuous compared with a discontinuous negative pressure needle aspiration system.
Conflict of interest statement
Peter von Dadelszen is the first author of one of the trials (von Dadelszen 2005) included in this review. Zarko Alfirevic is the principal investigator of the study which was excluded (Cochrane 2003).
Figures






































































Update of
-
Instruments for chorionic villus sampling for prenatal diagnosis.Cochrane Database Syst Rev. 2003;(1):CD000114. doi: 10.1002/14651858.CD000114. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2013 Jan 31;(1):CD000114. doi: 10.1002/14651858.CD000114.pub2. PMID: 12535386 Updated.
References
References to studies included in this review
Barkai 1989 {published data only}
-
- Barkai G, Rabinovici, Chaki R, Shalev J, Katznelson MBM, Mashiach S, et al. Transcervical chorionic villi sampling: a comparison between the silver cannula and the portex catheter. Gynecologic and Obstetric Investigation 1989;27:70‐3. - PubMed
-
- Rabinovici J, Barkai G, Maschiach S, Chaki R, Goldman B. Transcervical CVS. A comparison between the Portex canula and the silver needle. Proceedings of 11th European Congress of Perinatal Medicine; 1988 April 10‐13; Rome, Italy. 1988:143.
Battagliarin 2009 {published data only}
-
- Battagliarin G, Lanna M, Coviello D, Tassis B, Quarenghi A, Nicolini U. A randomized study to assess two different techniques of aspiration while performing transabdominal chorionic villus sampling. Ultrasound in Obstetrics and Gynecology 2009;33(2):169‐72. - PubMed
Buyukkurt 2010 {published data only}
-
- Buyukkurt S, Seydaoglu G, Demir C, Ozgunen FT, Evruke C, Guzel AB, et al. Evaluation of the feasibility of a new method for performing chorion villus sampling. Clinical and Experimental Obstetrics & Gynecology 2010;37(3):190‐2. - PubMed
Chalkiadakis 1993 {published data only}
-
- Chalkiadakis G, Menton M, Wiest E, Schrage R. Catheter guided chorionic villus sampling technique. A prospective randomised study [Sondergesteurte Choriozottenbiopsietechnik ‐ eine prospektive randomisierte Studie]. Archives of Gynecology and Obstetrics 1993;254(1‐4):1243‐4.
MacKenzie 1986 {published data only}
-
- MacKenzie WE, Holmes DS, Webb T, Whitehouse C, Newton JR. A randomized study of three cannulas for transcervical chorionic villus sampling. American Journal of Obstetrics and Gynecology 1986;154:34‐9. - PubMed
Pons 1989 {published data only}
-
- Pons JC, Fernandez H, Eydoux P, Diallo A, Doumerc S, Frydman R, et al. Chorionic villus sampling (CVS). Randomized study of efficacy of two transcervical biopsy methods: aspiration cannulas and small forceps. European Journal of Obstetrics and Gynecology and Reproductive Biology 1989;32:187‐94. - PubMed
von Dadelszen 2005 {published and unpublished data}
-
- Dadelszen P, Sermer M, Hillier J, Allen L, Fernandes B, Johnson J, et al. Randomised‐controlled trial (RCT) of instruments for transcervical chorionic villus sampling (CVS). American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S187.
-
- Dadelszen P, Sermer M, Hillier J, Allen L, Fernandes B, Johnson J, et al. A randomised controlled trial of biopsy forceps and cannula aspiration for transcervical chorionic villus sampling. BJOG: an international journal of obstetrics and gynaecology 2005;112:559‐66. - PubMed
References to studies excluded from this review
Cochrane 2003 {published data only}
-
- Cochrane L, Ainscough M, Alfirevic Z. The influence of needle and syringe size on chorionic villus sampling of term placentae: a randomised trial. Prenatal Diagnosis 2003;23:1049‐51. - PubMed
Additional references
Alfirevic 1999
Buyukkurt 2009
-
- Buyukkurt S, Evruke C, Demir C, Ozgunen FT, Kadayifci O. A new device to facilitate the chorion villus sampling. Journal of Perinatal Medicine 2009;37:425. - PubMed
CEMAT 1998
-
- CEMAT Group. Randomised trial to assess safety and fetal outcome of early and midtrimester amniocentesis. The Canadian Early and Mid‐trimester Amniocentesis Trial (CEMAT) Group. Lancet 1998;351(9098):242‐7. - PubMed
Clarke 2000
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
RevMan 2000 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Smidt‐Jensen 1992
-
- Smidt‐Jensen S, Permin M, Philip J, Lundsteen C, Zachary JM, Fowler SE, et al. Randomised comparison of amniocentesis and transabdominal and transcervical chorionic villus sampling. Lancet 1992;340:1237‐44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

