Ginkgo biloba extract for age-related macular degeneration
- PMID: 23440785
- PMCID: PMC7061350
- DOI: 10.1002/14651858.CD001775.pub2
Ginkgo biloba extract for age-related macular degeneration
Abstract
Background: Ginkgo is used in the treatment of peripheral vascular disease and 'cerebral insufficiency'. It is thought to have several potential mechanisms of action including increased blood flow, platelet activating factor antagonism, and prevention of membrane damage caused by free radicals. Vascular factors and oxidative damage are thought to be two potential mechanisms in the pathology of age-related macular degeneration (AMD).
Objectives: The objective of this review was to determine the effect of Ginkgo biloba extract on the progression of AMD.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2012, Issue 10), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2012), EMBASE (January 1980 to October 2012), Allied and Complementary Medicine Database (AMED) (January 1985 to October 2012), OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 5 October 2012. We searched the reference lists of identified reports and the Science Citation Index. We also contacted investigators of included studies for additional information.
Selection criteria: All randomised trials in people with AMD where Ginkgo biloba extract had been compared to control were included.
Data collection and analysis: The review author extracted data using a standardised form. The data were verified with the trial investigators. Trial quality was assessed.
Main results: Two published trials were identified that randomised a total of 119 people. In one study conducted in France, 20 people were randomly allocated to Gingko biloba extract EGb 761 80 mg twice daily or placebo. In the other study conducted in Germany, 99 people were randomly allocated to two different doses of Ginkgo biloba extract EGb 761 (240 mg per day and 60 mg per day). Treatment duration in both studies was six months. Both trials reported some positive effects of Ginkgo biloba on vision however their results could not be pooled. Adverse effects and quality of life for people with AMD were not reported.
Authors' conclusions: The question as to whether people with AMD should take Ginkgo biloba extract to prevent progression of the disease has not been answered by research to date. Two small trials have suggested possible benefit of Gingko biloba on vision and further trials are warranted. Ginkgo biloba is widely used in China, Germany, and France. Future trials should be larger, and last longer, in order to provide a more robust measure of the effect of Gingko biloba extract on AMD.
Conflict of interest statement
None known.
Figures
Update of
-
Ginkgo biloba extract for age-related macular degeneration.Cochrane Database Syst Rev. 2000;(2):CD001775. doi: 10.1002/14651858.CD001775. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2013 Jan 31;(1):CD001775. doi: 10.1002/14651858.CD001775.pub2. PMID: 10796819 Updated.
References
References to studies included in this review
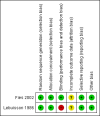
Fies 2002 {published data only}
-
- Fies P, Dienel A. Ginkgo extract in impaired vision‐‐treatment with special extract EGb 761 of impaired vision due to dry senile macular degeneration. Wiener Medizinische Wochenschrift 2002;152(15‐16):423‐6. - PubMed
Lebuisson 1986 {published data only}
-
- Lebuisson DA, Leroy L, Rigal G. Treatment of senile macular degeneration with Ginkgo biloba extract. A preliminary double‐blind drug vs. placebo study [Traitement des degenerescences "maculaires seniles" par l'extrait de Ginkgo biloba: etude preliminaire a double insu face au placebo]. La Press Medicale 1986;15(31):1556‐8. - PubMed
Additional references
Birks 2009
-
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003120.pub3] - DOI
Droy‐Lefaix 1993
-
- Droy‐Lefaix MT, Szabo ME, Doly M. Ischaemia and reperfusion‐induced injury in rat retina obtained from normotensive and spontaneously hypertensive rates: effects of free radical scavengers. International Journal of Tissue Reactions 1993;15(2):85‐91. - PubMed
Droy‐Lefaix 1995
-
- Droy‐Lefaix MT, Cluzel J, Menerath JM, Bonhomme B, Doly M. Antioxidant effect of a ginkgo biloba extract (EGb 761) on the retina. International Journal of Tissue Reactions 1995;17(3):93‐100. - PubMed
Evans 1996
-
- Evans JR, Rooney C, Dattani N, Ashwood F, Wormald RPL. Causes of blindness and partial sight in England and Wales. Health Trends 1996;28:5‐12.
Glanville 2006
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, 2011.
Hilton 2004
Kleijnen 1992
-
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet 1992;340(8828):1136‐9. - PubMed
Klein 1992
-
- Klein R, Klein BEK, Linton KL. Prevalence of age‐related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99(6):933‐43. - PubMed
Knipschild 1998
-
- Knipschild PG, Hoerr R, Oschmann R, Rossum E, Dongen MCJM. Optimization of placebos for double‐blind clinical trials: experience with a phytopharmaceutical. Arzneimittelforschung 1998;48(10):1033‐6. - PubMed
Pritz‐Hohmeier 1994
-
- Pritz‐Hohmeier S, Chao TI, Krenzlin J, Reichenbach A. Effect of in vivo application of the Ginkgo biloba extract EGb 761 (Rokan) on the susceptibility of mammalian retinal cells to proteolytic enzymes. Ophthalmic Research 1994;26(2):80‐6. - PubMed
Rosenblatt 1997
-
- Rosenblatt M, Mindel J. Spontaneous hyphema associated with ingestion of Ginkgo biloba extract. New England Journal of Medicine 1997;336(15):1108. - PubMed
Rowin 1996
-
- Rowin J, Lewis SL. Spontaneous bilateral subdural hematomas associated with chronic Ginkgo biloba ingestion. Neurology 1996;46(6):1775‐6. - PubMed
References to other published versions of this review
Evans 1999
-
- Evans JR. Ginkgo biloba extract for. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001775] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials