Treating BCG-induced disease in children
- PMID: 23440826
- PMCID: PMC6532703
- DOI: 10.1002/14651858.CD008300.pub2
Treating BCG-induced disease in children
Abstract
Background: Bacillus Calmette-Guerín (BCG) is a live attenuated vaccine to prevent tuberculosis, routinely administered at birth as part of the World Health Organization global expanded immunisation programme. Given intradermally, it can cause adverse reactions, including local, regional, distant and disseminated manifestations that may cause parental distress. Rarely, it can cause serious illness and even death. Among those patients with immunocompromised conditions, such as the human immunodeficiency virus (HIV) infection, the complication rate is even higher.
Objectives: To assess the effects of different interventions for treating BCG-induced disease in children.
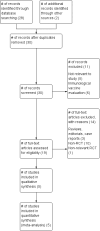
Search methods: The following databases were searched: the Cochrane Infectious Diseases Group Specialized Register and Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (The Cochrane Library 2012, Issue 4); MEDLINE (1966 to November 2012); EMBASE (1947 to November 2012); and LILACS (1980 to November 2012). The metaRegister of Controlled Trials (mRCT) and the WHO trials search portal. Conference proceedings for relevant abstracts and experts were also contacted to identify studies. No language restrictions were applied.
Selection criteria: Randomized controlled trials (RCTs) comparing any medical or surgical treatment modality for BCG-induced disease in children.
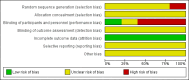
Data collection and analysis: Two authors independently evaluated titles, applied inclusion criteria, and assessed the risk of bias of studies. The primary outcomes were the failure rate of therapies for all types of BCG vaccine-induced complications and the time to resolution of illness measured in months. The secondary outcomes were death from BCG vaccine-induced disease and the all-cause mortality. Risk ratios (RRs) were used as measure of effect for dichotomous outcomes and mean differences for continuous outcomes.
Main results: Five RCTs analysing 341 children addressed the primary outcomes and were included. Four arms compared oral antibiotics to no intervention or placebo, one arm evaluated needle aspiration compared to no intervention, and another evaluated the use of locally instilled isoniazid versus oral erythromycin.Two small studies evaluated oral isoniazid; we are uncertain of whether this intervention has an effect on clinical failure (RR 1.48; 95% Confidence Interval (CI) 0.79 to 2.78; 54 participants, two studies, very low quality evidence). Similarly, for oral erythromycin, we are uncertain if there is an effect (clinical failure RR 1.03; 95% CI 0.70 to 1.53; 148 participants, three studies, very low quality evidence), and for oral isoniazid plus rifampicin (clinical failure, RR 1.20; 95% CI 0.51 to 2.83; 35 participants, one study, very low quality evidence).In patients with lymphadenitis abscess, needle aspiration may reduce clinically persistent BCG-induced disease at 6 to 9 months of follow-up (RR 0.13; 95% CI 0.03 to 0.55; 77 participants, one study, low quality evidence). In another study of patients with the same condition, aspiration plus local instillation of isoniazid reduces time to clinical cure compared to aspiration plus oral erythromycin (mean difference 1.49 months less; 95% CI 0.82 to 2.15 less; 27 participants, one study).No RCTs of HIV-infected infants with a BCG-induced disease evaluated the use of antibiotics or other therapies for reducing the rate of clinical failure or the time to clinical resolution. No data on mortality secondary to the interventions for treating BCG-induced disease were reported.
Authors' conclusions: It is unclear if oral antibiotics (isoniazid, erythromycin, or a combination of isoniazid plus rifampicin) are effective for the resolution of BCG-induced disease. Most non-suppurated lymphadenitis will resolve without treatment in 4 to 6 months. Patients with lymphadenitis abscess might benefit from needle aspiration and possibly local instillation of isoniazid could shorten recovery time. Included studies were generally small and could be better conducted. Further research should evaluate the use of needle aspiration and local instillation of isoniazid in fluctuant nodes. Therapeutic and preventive measures in HIV-infected infants could be important given the higher risk of negative outcomes in this group.
Conflict of interest statement
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, or expert testimony).
Figures


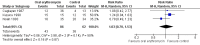
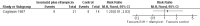
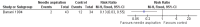






Update of
- doi: 10.1002/14651858.CD008300
References
References to studies included in this review
Banani 1994 {published data only (unpublished sought but not used)}
Caglayan 1987 {published data only (unpublished sought but not used)}
-
- Caglayan S, Yegin O, Kayran K, Timocin N, Kasirga E, Gun M. Is medical therapy effective for regional lymphadenitis following BCG vaccination?. American Journal of Diseases of Children 1987;141(11):1213‐4. - PubMed
Close 1985 {published data only (unpublished sought but not used)}
-
- Close GC, Nasiiro R. Management of BCG adenitis in infancy. Journal of Tropical Pediatrics 1985;31(5):286. - PubMed
Kuyucu 1998 {published data only (unpublished sought but not used)}
-
- Kuyucu N, Kuyucu S, Ocal B, Tezic T. Comparison of oral erythromycin, local administration of streptomycin and placebo therapy for nonsuppurative Bacillus Calmette‐Guerin lymphadenitis. The Pediatric Infectious Disease Journal 1998;17(6):524‐5. - PubMed
Noah 1993 {published data only (unpublished sought but not used)}
-
- Noah PK, Pande D, Johnson B, Ashley D. Evaluation of oral erythromycin and local isoniazid instillation therapy in infants with Bacillus Calmette‐Guerin lymphadenitis and abscesses. The Pediatric Infectious Disease Journal 1993;12(2):136‐9. - PubMed
References to studies excluded from this review
Amato Neto 1973 {published data only}
-
- Amato Neto V, Oliveira e Silva YK, Finger H, Bianchi A, do Amaral JP, Cardozo BS, et al. Analysis of the adverse effects occurring after the administration of BCG by oral route in 5,579 healthy children [Análise das manifestações colaterais decorrentes da administração da vacina BCG, por via oral, a 5,579 crianças sadias]. Revista Paulista De Medicina 1973;82(3):135‐8. - PubMed
Au 1978 {published data only}
-
- Au FC, Webber B, Rosenberg SA. Pulmonary granulomas induced by BCG. Cancer 1978;41(6):2209‐14. - PubMed
Balkan 1992 {published data only (unpublished sought but not used)}
-
- Balkan E, Sapan N, Unal M, Dogruyol H. Treatment of the suppurative BCG lymphadenitis [Süpüre BCG Lenfadenit Tedavisi]. Pediatrik Cerrahi Dergisi 1992;6:29‐32.
Bhandari 1980 {published data only (unpublished sought but not used)}
-
- Bhandari B, Khurana R, Mandowara SL. Management of post‐BCG lymphadenitis. Indian Journal of Pediatrics 1980;47(388):367‐70. - PubMed
Caglayan 1991 {published data only (unpublished sought but not used)}
-
- Caglayan S, Arikan A, Yaprak I, Aksoz K, Kansoy S. Management of suppuration in regional lymph nodes secondary to BCG vaccination. Acta Paediatrica Japonica 1991;33(6):699‐702. - PubMed
Cerda de Palou 2003 {published data only}
-
- Cerdá de Palou E, Santy HP. Lymphadenitis as a complication following vaccination with Bacillus Calmette‐Guérin (BCG). Nederlands Tijdschrift voor Geneeskunde 2003;147(12):569‐72. - PubMed
Chavganc 1969 {published data only}
De Britto 1997 {published data only (unpublished sought but not used)}
-
- Britto RL, Ramanathan VD, Gupte MD. Regional lymphadenitis following antileprosy vaccine BCG with killed Mycobacterium leprae. International journal of leprosy and other mycobacterial diseases 1997;65(1):12‐9. - PubMed
Easton 1984 {published data only (unpublished sought but not used)}
-
- Easton PA, Hershfield ES. Lymphadenitis as a late complication of BCG vaccination. Tubercle 1984;65(3):205‐8. - PubMed
Ferreira 1996 {published data only (unpublished sought but not used)}
-
- Ferreira AA, Bunn‐Moreno MM, Sant'Anna CC, Ferreira MF. BCG vaccination in low birth weight newborns: analysis of lymphocyte proliferation, IL‐2 generation and intradermal reaction to PPD. Tubercle and Lung Disease 1996;77(5):476‐81. - PubMed
Goraya 2001 {published and unpublished data}
-
- Goraya JS, Virdi VS. Treatment of Calmette‐Guérin bacillus adenitis: a metaanalysis. The Pediatric Infectious Disease Journal 2001;20(6):632‐4. - PubMed
Guld 1955 {published data only (unpublished sought but not used)}
Gur 2002 {published data only (unpublished sought but not used)}
-
- Gür E, Arvas A. Dose of Calmette‐Guérin bacillus vaccination in infants. The Pediatric Infectious Disease Journal 2002;21(4):359‐60. - PubMed
Hanley 1985 {published data only (unpublished sought but not used)}
Hengster 1997 {published data only (unpublished sought but not used)}
-
- Hengster P, Sölder B, Fille M, Menardi G. Surgical treatment of bacillus Calmette Guérin lymphadenitis. World Journal of Surgery 1997;21(5):520‐3. - PubMed
Kendrick 1981 {published data only (unpublished sought but not used)}
-
- Kendrick MA, Comstock GW. BCG vaccination and the subsequent development of cancer in humans. Journal of the National Cancer Institute 1981;66(3):431‐7. - PubMed
Koivisto 1974 {published data only}
-
- Koivisto M, Brander E, Hakosalo J, Wasz‐Höckert O. Comparative study of 3 BCG vaccines [Kolmen BCG‐rokotteen vertaileva tutkimus]. Duodecim 1974;90(24):1717‐22. - PubMed
Lachmann 1976 {published data only (unpublished sought but not used)}
-
- Lachmann D, Howanietz L. Clinical aspect and therapy of BCG‐lymphadenitis (author's transl). Pädiatrie und Pädologie 1976;11(1):283‐6. - PubMed
Mitinskaia 1994 {published data only}
-
- Mitinskaia LA, Lukhimenko NV, Kamaeva VF. BCG vaccination and increasing the effectiveness of treatment of post‐vaccination complications by the use of rifampicin and dimexide. Problemy Tuberkuleza 1994;5:4‐7. - PubMed
Nazir 2005 {published data only (unpublished sought but not used)}
-
- Nazir Z, Qazi SH. Bacillus Calmette‐Guerin (BCG) lymphadenitis‐changing trends and management. Journal of Ayub Medical College, Abbottabad: JAMC 2005;17(4):16‐8. - PubMed
Oguz 1992 {published data only (unpublished sought but not used)}
-
- Oğuz F, Müjgan S, Alper G, Alev F, Neyzi O. Treatment of Bacillus Calmette‐Guérin‐associated lymphadenitis. The Pediatric Infectious Disease Journal 1992;11(10):887‐8. - PubMed
Rabie 2011 {published data only}
-
- Rabie H, Violari A, Duong T, Madhi SA, Josipovic D, Innes S, et al. Early antiretroviral treatment reduces risk of bacille Calmette‐Guérin immune reconstitution adenitis. The International Journal of Tuberculosis and Lung Disease: the official journal of the International Union against Tuberculosis and Lung Disease 2011;15(9):1194‐200. - PMC - PubMed
Samileh 2006 {published data only (unpublished sought but not used)}
-
- Samileh N, Ahmad S, Farzaneh A, Shahnaz R, Lida F, Mohammad N. Immunity status in children with Bacille Calmette‐Guerin adenitis. A prospective study in Tehran, Iran. Saudi Medical Journal 2006;27(11):1719‐24. - PubMed
Teulieres 1991 {published data only (unpublished sought but not used)}
-
- Teulieres L, Diouf MA, Chaud P, Saint‐Cyr A, Saliou P. Comparative trial of administration of half (0.05 mg) and quarter (0.025 mg) dose of intradermal Pasteur BCG on 291 infants from birth to 1 year in French Guyana. Vaccine 1991;9(7):521‐4. - PubMed
Thayyil‐Sudhan 1999 {published data only (unpublished sought but not used)}
Yuan 2001 {published data only (unpublished sought but not used)}
-
- Yuan Y, Wu M, Dai X, Ni Z, Su G. Study on different treatment methods of tuberculous lymphadenitis caused by BCG vaccination. Journal of Clinical Internal Medicine 2001;18(3):226.
Additional references
Bolger 2006
Bonnlander 1993
Deeks 2005
-
- Deeks SL, Clark M, Scheifele DW. Serious adverse events associated with Bacille Calmette‐Guérin vaccine in Canada. Pediatric Infectious Disease Journal 2005;24(6):538‐41. - PubMed
Fine 1999
-
- Fine P, Carneiro I, Milstein JB, Clements CJ. BCG immunization policies. Issues relating to the use of BCG in immunisation programmes. Geneva: World Health Organization (WHO/V&B/99.23) 1999.
FitzGerald 2000
-
- Fitzgerald JM. Management of adverse reactions to Bacille Calmette‐Guérin vaccine. Clinical Infectious Diseases 2000;31(Suppl 3):S75‐6. - PubMed
French 2012
-
- French MAH. Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on. The Medical Journal of Australia 2012;196(5):318‐21. - PubMed
Goraya 2002
Hatherill 2011
-
- Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Archives of Disease in Childhood 2011;96(9):851‐6. - PubMed
Hesselberg 1972
Hesseling 2004
-
- Hesseling AC, Schaaf HS, Victor T, Beyers N, Marais BJ, Cotton MF, et al. Resistant Mycobacterium bovis Bacillus Calmette‐Guérin disease: implications for management of Bacillus Calmette‐Guérin Disease in human immunodeficiency virus‐infected children. Pediatric Infectious Disease Journal 2004;23(5):476‐9. - PubMed
Hesseling 2006
-
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, et al. Bacille Calmette‐Guérin Vaccine‐Induced Disease in HIV‐Infected and HIV‐Uninfected Children. Clinical Infectious Diseases 2006;42(4):548‐58. - PubMed
Hesseling 2007
-
- Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey‐Faussett P, et al. The risk of disseminated Bacille Calmette‐Guerin (BCG) disease in HIV‐infected children. Vaccine 2007;25(1):14‐8. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. 2011.
Karpelowsky 2008
-
- Karpelowsky JS, Alexander AG, Dix Peek S, Millar AJW, Rode H. Surgical complications of bacille Calmette‐Guérin (BCG) infection in HIV‐infected children: Time for a change in policy?. South African Medical Journal 2008;98(10):801‐4. - PubMed
Lotte 1984
-
- Lotte A, Wasz‐Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E. BCG complications: estimates of risks among vaccinated subjects and statistical analysis of their main characteristics. Advances in Tuberculosis Research 1984;21:107‐93. - PubMed
Lotte 1988
-
- Lotte A, Dam HG, Henderson R. Second IUATLD study on complications induced by intradermal BCG vaccination. Bulletin of the International Union Against Tuberculosis and Lung Disease 1988;63(2):47‐83. - PubMed
Milstien 1990
Milstien 1993
-
- Milstien JB. Immunological Basis for Immunization / Module 5: Tuberculosis. (WHO/EPI/GEN/93.15). Geneva: World Health Organization 1993.
Nutall 2011
Nuttall 2008
-
- Nuttall JJ, Davies MA, Hussey GD, Eley BS. Bacillus Calmette‐Guérin (BCG) vaccine‐induced complications in children treated with highly active antiretroviral therapy. International Journal of Infectious Diseases 2008;12(6):e99‐105. - PubMed
O'Brien 1995
-
- O'Brien KL, Ruff AJ, Louis MA, Desormeaux J, Joseph DJ, McBrien M, et al. Bacillus Calmette‐Guérin complications in children born to HIV‐1‐infected women with a review of the literature. Pediatrics 1995;95(3):414‐8. - PubMed
Rabie 2008
-
- Rabie H, Violari A, Madhi S, Gibb D, Steyn J, Niekerk R, et al. Complications of BCG Vaccination in HIV‐infected and uninfected Children: CHER Study. Proceedings of the 15th Conference on Retroviruses and Opportunistic Infections. Boston: Foundation for Retrovirology and Human Health, 2008.
Ritz 2009
Sharma 2011
Talbot 1997
-
- Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette‐Guérin disease after vaccination: case report and review. Clinical Infectious Diseases 1997;24(6):1139‐46. - PubMed
WHO 2007
-
- World Health Organization. Revised BCG vaccination guidelines for infants at risk for HIV infection. The Weekly Epidemiological Record 2007;82(21):193‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

