Glutamatergic modulation of synaptic-like vesicle recycling in mechanosensory lanceolate nerve terminals of mammalian hair follicles
- PMID: 23440964
- PMCID: PMC3678041
- DOI: 10.1113/jphysiol.2012.243659
Glutamatergic modulation of synaptic-like vesicle recycling in mechanosensory lanceolate nerve terminals of mammalian hair follicles
Abstract
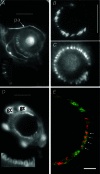
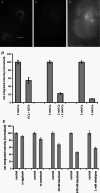
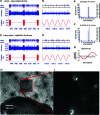
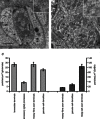
Our aim in the present study was to determine whether a glutamatergic modulatory system involving synaptic-like vesicles (SLVs) is present in the lanceolate ending of the mouse and rat hair follicle and, if so, to assess its similarity to that of the rat muscle spindle annulospiral ending we have described previously. Both types of endings are formed by the peripheral sensory terminals of primary mechanosensory dorsal root ganglion cells, so the presence of such a system in the lanceolate ending would provide support for our hypothesis that it is a general property of fundamental importance to the regulation of the responsiveness of the broad class of primary mechanosensory endings. We show not only that an SLV-based system is present in lanceolate endings, but also that there are clear parallels between its operation in the two types of mechanosensory endings. In particular, we demonstrate that, as in the muscle spindle: (i) FM1-43 labels the sensory terminals of the lanceolate ending, rather than the closely associated accessory (glial) cells; (ii) the dye enters and leaves the terminals primarily by SLV recycling; (iii) the dye does not block the electrical response to mechanical stimulation, in contrast to its effect on the hair cell and dorsal root ganglion cells in culture; (iv) SLV recycling is Ca(2+) sensitive; and (v) the sensory terminals are enriched in glutamate. Thus, in the lanceolate sensory ending SLV recycling is itself regulated, at least in part, by glutamate acting through a phospholipase D-coupled metabotropic glutamate receptor.
Figures




References
-
- Albani-Torregrossa S, Attucci S, Marinozzi M, Pellicciari R, Moroni F, Pellegrini-Giampietro DE. Antagonist pharmacology of metabotropic glutamate receptors coupled to phospholipase D activation in adult rat hippocampus: focus on (2R,1′S,2′R,3′S)-2-(2′-carboxy-3′-phenylcyclopropyl) glycine versus 3,5-dihydroxyphenylglycine. Mol Pharmacol. 1999;55:699–707. - PubMed
-
- Andres KH. Über die Feinstruktur des Rezeptoren an Sinushaaren. Z Zellforsch Microsc Anat. 1966;75:339–365. - PubMed
-
- Banks RW, Bewick GS, Reid B, Richardson C. Evidence for activity-dependent modulation of sensory-terminal excitability in spindles by glutamate release from synaptic-like vesicles. Adv Exp Med Biol. 2002;508:13–18. - PubMed
-
- Bannister LH. Sensory terminals of peripheral nerves. In: Landon DH, editor. The Peripheral Nerve. London: Chapman and Hall; 1976. pp. 369–463.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

