RNA interference by feeding in vitro-synthesized double-stranded RNA to planarians: methodology and dynamics
- PMID: 23441014
- PMCID: PMC3909682
- DOI: 10.1002/dvdy.23950
RNA interference by feeding in vitro-synthesized double-stranded RNA to planarians: methodology and dynamics
Abstract
Background: The ability to assess gene function is essential for understanding biological processes. Currently, RNA interference (RNAi) is the only technique available to assess gene function in planarians, in which it has been induced by means of injection of double-stranded RNA (dsRNA), soaking, or ingestion of bacteria expressing dsRNA.
Results: We describe a simple and robust RNAi protocol, involving in vitro synthesis of dsRNA that is fed to the planarians. Advantages of this protocol include the ability to produce dsRNA from any vector without subcloning, resolution of ambiguities in quantity and quality of input dsRNA, as well as time and ease of application. We have evaluated the logistics of inducing RNAi in planarians using this methodology in careful detail, from the ingestion and processing of dsRNA in the intestine, to timing and efficacy of knockdown in neoblasts, germline, and soma. We also present systematic comparisons of effects of amount, frequency, and mode of dsRNA delivery.
Conclusions: This method gives robust and reproducible results and is amenable to high-throughput studies. Overall, this RNAi methodology provides a significant advance by combining the strengths of current protocols available for dsRNA delivery in planarians and has the potential to benefit RNAi methods in other systems.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
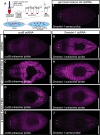
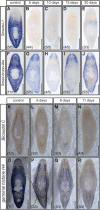


Similar articles
-
RNA Interference in Planarians: Feeding and Injection of Synthetic dsRNA.Methods Mol Biol. 2018;1774:455-466. doi: 10.1007/978-1-4939-7802-1_18. Methods Mol Biol. 2018. PMID: 29916171
-
Systemic RNA Interference in Planarians by Feeding of dsRNA Containing Bacteria.Methods Mol Biol. 2018;1774:445-454. doi: 10.1007/978-1-4939-7802-1_17. Methods Mol Biol. 2018. PMID: 29916170
-
RNAi Screening to Assess Tissue Regeneration in Planarians.Methods Mol Biol. 2022;2450:509-527. doi: 10.1007/978-1-0716-2172-1_27. Methods Mol Biol. 2022. PMID: 35359326 Free PMC article.
-
Delivery of dsRNA for RNAi in insects: an overview and future directions.Insect Sci. 2013 Feb;20(1):4-14. doi: 10.1111/j.1744-7917.2012.01534.x. Epub 2012 Jul 5. Insect Sci. 2013. PMID: 23955821 Review.
-
Strategies for enhancing the efficiency of RNA interference in insects.Pest Manag Sci. 2021 Jun;77(6):2645-2658. doi: 10.1002/ps.6277. Epub 2021 Feb 1. Pest Manag Sci. 2021. PMID: 33440063 Review.
Cited by
-
The Small RNA Universe of Capitella teleta.Front Mol Biosci. 2022 Feb 25;9:802814. doi: 10.3389/fmolb.2022.802814. eCollection 2022. Front Mol Biosci. 2022. PMID: 35281272 Free PMC article.
-
Heterotrimeric G proteins regulate planarian regeneration and behavior.Genetics. 2023 Apr 6;223(4):iyad019. doi: 10.1093/genetics/iyad019. Genetics. 2023. PMID: 36763503 Free PMC article.
-
Feeding exogenous dsRNA interferes with endogenous sRNA accumulation in Paramecium.DNA Res. 2020 Feb 1;27(1):dsaa005. doi: 10.1093/dnares/dsaa005. DNA Res. 2020. PMID: 32339224 Free PMC article.
-
RNA Interference (RNAi) Screening in Drosophila.Genetics. 2018 Mar;208(3):853-874. doi: 10.1534/genetics.117.300077. Genetics. 2018. PMID: 29487145 Free PMC article.
-
The pharyngeal nervous system orchestrates feeding behavior in planarians.Sci Adv. 2020 Apr 8;6(15):eaaz0882. doi: 10.1126/sciadv.aaz0882. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32285000 Free PMC article.
References
-
- Baguñà J. Mitosis in the intact and regenerating planarian Dugesia mediterranea n.sp. I. Mitotic studies during growth, feeding and starvation. J. Exp. Zool. 1976;195:53–64.
-
- Barnes WM, Rowlyk KR. Magnesium precipitate hot start method for PCR. Mol Cell Probes. 2002;16:167–171. - PubMed
-
- Bowen ID, Ryder TA, Thompson JA. The fine structure of the planarian Polycelis tenuis Iijima. II. The intestine and gastrodermal phagocytosis. Protoplasma. 1974;79:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

