AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
- PMID: 23441028
- PMCID: PMC3579147
- DOI: 10.4093/dmj.2013.37.1.1
AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
Erratum in
- Diabetes Metab J. 2013 Apr;37(2):155
Abstract
AMPK is an evolutionary conserved sensor of cellular energy status that is activated during exercise. Pharmacological activation of AMPK promotes glucose uptake, fatty acid oxidation, mitochondrial biogenesis, and insulin sensitivity; processes that are reduced in obesity and contribute to the development of insulin resistance. AMPK deficient mouse models have been used to provide direct genetic evidence either supporting or refuting a role for AMPK in regulating these processes. Exercise promotes glucose uptake by an insulin dependent mechanism involving AMPK. Exercise is important for improving insulin sensitivity; however, it is not known if AMPK is required for these improvements. Understanding how these metabolic processes are regulated is important for the development of new strategies that target obesity-induced insulin resistance. This review will discuss the involvement of AMPK in regulating skeletal muscle metabolism (glucose uptake, glycogen synthesis, and insulin sensitivity).
Keywords: AMPK; Exercise; Glucose uptake; Insulin resistance; Obesity.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
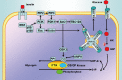

References
-
- Dyck JR, Gao G, Widmer J, Stapleton D, Fernandez CS, Kemp BE, Witters LA. Regulation of 5'-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits. J Biol Chem. 1996;271:17798–17803. - PubMed
-
- Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, Seidman JG, Ingwall JS, Balschi JA, Tian R. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–1439. - PMC - PubMed
-
- Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5'-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. - PubMed
-
- Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for gamma3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E194–E200. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

