Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury
- PMID: 23441106
- PMCID: PMC3580988
Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury
Abstract
Background: Because oxidative stress is assumed to be a key mechanism in the pathological process of age-related macular degeneration (AMD), increasing numbers of studies have focused on discovering new pathways and treatments for reducing oxidative damage. Our work investigates the potential role of the cannabinoid receptor 1 (CB1) in oxidative stress of primary human retinal pigment epithelial (RPE) cells, a cellular model of AMD.
Methods: Primary human RPE cells were cultured and exposed to hydrogen peroxide for 24 h to induce oxidative damage. The expression of and changes in the CB1 receptor were determined with western blot assay and confocal imaging. The CB1 receptor in the RPE cells was inhibited with small interfering RNA (siRNA) or rimonabant (SR141716). Cell viability, apoptosis, and reactive oxygen species production were measured by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and sulforhodamine B assay, annexin V and propidium iodide staining, and the dichlorofluorescein fluorescence assay, respectively. Intracellular superoxide dismutase activity was assayed with a commercially available assay kit. Phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) protein expression and activation of signaling molecules were assessed with western blot analysis.
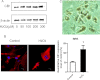
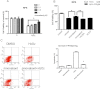
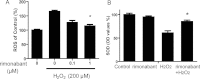
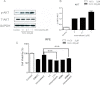
Results: We showed that human RPE cells express the CB1 receptor. In addition, oxidative stress upregulates the expression of the CB1 receptor. Deleting the CB1 receptor or treating with the CB1 receptor antagonist rimonabant (SR141716) rescued RPE cells from hydrogen peroxide-induced oxidative damage. Rimonabant pretreatment effectively reduced the apoptosis of RPE cells, inhibited the generation of intracellular reactive oxygen species and elevated the activity of superoxide dismutase. In addition, rimonabant significantly strengthened the oxidative stress-induced activation of the PI3K/Akt signaling pathway.
Conclusions: The results demonstrate the expression and regulation of CB1 receptors in human RPE cells. Inhibiting the CB1 receptor may be an effective therapeutic strategy for AMD by downregulating oxidative stress signaling and facilitating PI3K/Akt activation.
Figures

Similar articles
-
Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells.Mol Vis. 2009 Jun 14;15:1243-51. Mol Vis. 2009. PMID: 19547718 Free PMC article.
-
Exendin-4 Protects Human Retinal Pigment Epithelial Cells from H2O2-Induced Oxidative Damage via Activation of NRF2 Signaling.Ophthalmic Res. 2020;63(4):404-412. doi: 10.1159/000504891. Epub 2019 Dec 20. Ophthalmic Res. 2020. PMID: 31865348
-
Squamosamide derivative FLZ protects retinal pigment epithelium cells from oxidative stress through activation of epidermal growth factor receptor (EGFR)-AKT signaling.Int J Mol Sci. 2014 Oct 17;15(10):18762-75. doi: 10.3390/ijms151018762. Int J Mol Sci. 2014. PMID: 25329617 Free PMC article.
-
Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases.Cell Mol Neurobiol. 2023 Oct;43(7):3265-3276. doi: 10.1007/s10571-023-01383-z. Epub 2023 Jul 1. Cell Mol Neurobiol. 2023. PMID: 37391574 Free PMC article. Review.
-
Oxidative stress induced cellular signaling in RPE cells.Front Biosci (Schol Ed). 2012 Jan 1;4(2):392-411. doi: 10.2741/s275. Front Biosci (Schol Ed). 2012. PMID: 22202067 Review.
Cited by
-
Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats.Int J Mol Sci. 2022 Dec 23;24(1):240. doi: 10.3390/ijms24010240. Int J Mol Sci. 2022. PMID: 36613692 Free PMC article.
-
Neuroprotection by (endo)Cannabinoids in Glaucoma and Retinal Neurodegenerative Diseases.Curr Neuropharmacol. 2018;16(7):959-970. doi: 10.2174/1570159X15666170724104305. Curr Neuropharmacol. 2018. PMID: 28738764 Free PMC article. Review.
-
The endocannabinoid system and ophthalmic pathologies: a review of molecular mechanisms and its implications for clinical practice.Front Med (Lausanne). 2025 Feb 5;12:1500179. doi: 10.3389/fmed.2025.1500179. eCollection 2025. Front Med (Lausanne). 2025. PMID: 39975680 Free PMC article. Review.
-
Expression and Function of the Endocannabinoid System in the Retina and the Visual Brain.Neural Plast. 2016;2016:9247057. doi: 10.1155/2016/9247057. Epub 2015 Dec 29. Neural Plast. 2016. PMID: 26839718 Free PMC article. Review.
-
Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide.Pharmaceuticals (Basel). 2025 Jan 17;18(1):117. doi: 10.3390/ph18010117. Pharmaceuticals (Basel). 2025. PMID: 39861178 Free PMC article.
References
-
- Javitt JC, Zhou Z, Maguire MG, Fine SL, Willke RJ. Incidence of exudative age-related macular degeneration among elderly Americans. Ophthalmology. 2003;110:1534–9. - PubMed
-
- Drobek-Słowik M, Karczewicz D, Safranow K. The potential role of oxidative stress in the pathogenesis of the age-related macular degeneration AMD. Postepy Hig Med Dosw (Online) 2007;61:28–37. - PubMed
-
- Martínez-Orgado J, Fernandez-Lopez D, Lizasoain I, Romero J. The seek of neuroprotection: introducing cannabinoids. Recent Pat CNS Drug Discov. 2007;2:131–9. - PubMed
-
- van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Micale V, Steardo L, Drago F, Iuvone T, Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–24. - PMC - PubMed
-
- Chen J, Matias I, Dinh T, Lu T, Venezia S, Nieves A, Woodward DF, Di Marzo V. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005;330:1062–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
