Transplantation of tissue-engineered human corneal endothelium in cat models
- PMID: 23441111
- PMCID: PMC3580986
Transplantation of tissue-engineered human corneal endothelium in cat models
Abstract
Purpose: To evaluate the performance of reconstructed tissue-engineered human corneal endothelium (TE-HCE) by corneal transplantation in cat models.
Methods: TE-HCE reconstruction was performed by culturing 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI)-labeled monoclonal HCE cells on denuded amniotic membranes (dAMs) in 20% fetal bovine serum-containing Dulbecco's Modified Eagle's Medium/Ham's Nutrient Mixture F12 (1:1) medium and 5% CO(2) at 37 ° C on a 24-well culture plate. The reconstructed TE-HCE was transplanted into cat corneas via lamellar keratoplasty with all of the endothelium and part of Descemet's membrane stripped. Postsurgical corneas were monitored daily with their histological properties examined during a period of 104 days after transplantation.
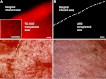
Results: The reconstructed TE-HCE at a density of 3,413.33 ± 111.23 cells/mm(2) in average established intense cell-cell and cell-dAM junctions. After lamellar keratoplasty surgery, no obvious edema was found in TE-HCE-transplanted cat corneas, which were transparent throughout the monitoring period. In contrast, intense corneal edema developed in dAM-transplanted cat corneas, which were turbid. The corneal thickness gradually decreased to 751.33 ± 11.37 μm on day 104 after TE-HCE transplantation, while that of dAM eye was over 1,000 μm in thickness during the monitoring period. A monolayer of endothelium consisting of TE-HCE-originated cells at a density of 2,573.33 ± 0.59 cells/mm(2) attached tightly to the surface of remnant Descemet's membrane over 104 days; this was similar to the normal eye control in cell density.
Conclusions: The reconstructed TE-HCE was able to function as a corneal endothelium equivalent and restore corneal function in cat models.
Figures





References
-
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–89. - PubMed
-
- Koizumi N, Sakamoto Y, Okumura N, Tsuchiya H, Torii R, Cooper LJ, Ban Y, Tanioka H, Kinoshita S. Cultivated corneal endothelial transplantation in a primate: possible future clinical application in corneal endothelial regenerative medicine. Cornea. 2008;27:S48–55. - PubMed
-
- Melles GR, Lander F, van Dooren BT, Pels E, Beekhuis WH. Preliminary clinical results of posterior lamellar keratoplasty through a sclerocorneal pocket incision. Ophthalmology. 2000;107:1850–6. - PubMed
-
- Price FW, Jr, Price MO. Descemet's stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–8. - PubMed
-
- Terry MA, Ousley PJ. Replacing the endothelium without corneal surface incisions or sutures: the first United States clinical series using the deep lamellar endothelial keratoplasty procedure. Ophthalmology. 2003;110:755–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
