Carbon turnover in the water-soluble protein of the adult human lens
- PMID: 23441119
- PMCID: PMC3580966
Carbon turnover in the water-soluble protein of the adult human lens
Abstract
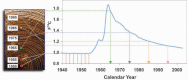
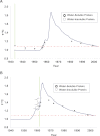
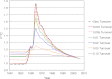
Purpose: Human eye lenses contain cells that persist from embryonic development. These unique, highly specialized fiber cells located at the core (nucleus) of the lens undergo pseudo-apoptosis to become devoid of cell nuclei and most organelles. Ostensibly lacking in protein transcriptional capabilities, it is currently believed that these nuclear fiber cells owe their extreme longevity to the perseverance of highly stable and densely packed crystallin proteins. Maintaining the structural and functional integrity of lenticular proteins is necessary to sustain cellular transparency and proper vision, yet the means by which the lens actually copes with a lifetime of oxidative stress, seemingly without any capacity for protein turnover and repair, is not completely understood. Although many years of research have been predicated upon the assumption that there is no protein turnover or renewal in nuclear fiber cells, we investigated whether or not different protein fractions possess protein of different ages by using the (14)C bomb pulse.
Methods: Adult human lenses were concentrically dissected by gently removing the cell layers in water or shaving to the nucleus with a curved micrometer-controlled blade. The cells were lysed, and the proteins were separated into water-soluble and water-insoluble fractions. The small molecules were removed using 3 kDa spin filters. The (14)C/C was measured in paired protein fractions by accelerator mass spectrometry, and an average age for the material within the sample was assigned using the (14)C bomb pulse.
Results: The water-insoluble fractions possessed (14)C/C ratios consistent with the age of the cells. In all cases, the water-soluble fractions contained carbon that was younger than the paired water-insoluble fraction.
Conclusions: As the first direct evidence of carbon turnover in protein from adult human nuclear fiber cells, this discovery supports the emerging view of the lens nucleus as a dynamic system capable of maintaining homeostasis in part due to intricate protein transport mechanisms and possibly protein repair. This finding implies that the lens plays an active role in the aversion of age-related nuclear (ARN) cataract.
Figures
References
-
- Beebe DC. “The Lens,” in Kaufman PL, Alm A. (Eds.), Adler’s Physiology of the Eye, Clinical Applications 10th Ed., Mosby, Inc., St. Louis, MO USA 2003; pp. 117–158.
-
- Taylor VL, Al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37:1396–410. - PubMed
-
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–85. - PubMed
-
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Exp Eye Res. 2005;80:709–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA093373/CA/NCI NIH HHS/United States
- 8P41GM103483/GM/NIGMS NIH HHS/United States
- R01ES002710/ES/NIEHS NIH HHS/United States
- P42ES004699/ES/NIEHS NIH HHS/United States
- R01 ES002710/ES/NIEHS NIH HHS/United States
- UL1RR024146/RR/NCRR NIH HHS/United States
- R01HG003352/HG/NHGRI NIH HHS/United States
- P42 ES004699/ES/NIEHS NIH HHS/United States
- 5P41RR013461/RR/NCRR NIH HHS/United States
- UL1 RR024146/RR/NCRR NIH HHS/United States
- R21EY018722/EY/NEI NIH HHS/United States
- R21 EY018722/EY/NEI NIH HHS/United States
- R01EY08747/EY/NEI NIH HHS/United States
- P41 RR013461/RR/NCRR NIH HHS/United States
- R01 EY008747/EY/NEI NIH HHS/United States
- P41 GM103483/GM/NIGMS NIH HHS/United States
- R01 HG003352/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
