The roles of telomerase in the generation of polyploidy during neoplastic cell growth
- PMID: 23441130
- PMCID: PMC3579318
- DOI: 10.1593/neo.121398
The roles of telomerase in the generation of polyploidy during neoplastic cell growth
Abstract
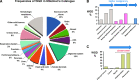
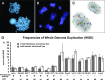
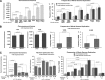
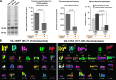
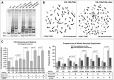
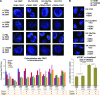
Polyploidy contributes to extensive intratumor genomic heterogeneity that characterizes advanced malignancies and is thought to limit the efficiency of current cancer therapies. It has been shown that telomere deprotection in p53-deficient mouse embryonic fibroblasts leads to high rates of polyploidization. We now show that tumor genome evolution through whole-genome duplication occurs in ∼15% of the karyotyped human neoplasms and correlates with disease progression. In a panel of human cancer and transformed cell lines representing the two known types of genomic instability (chromosomal and microsatellite), as well as the two known pathways of telomere maintenance in cancer (telomerase activity and alternative lengthening of telomeres), telomere dysfunction-driven polyploidization occurred independently of the mutational status of p53. Depending on the preexisting context of telomere maintenance, telomerase activity and its major components, human telomerase reverse transcriptase (hTERT) and human telomerase RNA component (hTERC), exert both reverse transcriptase-related (canonical) and noncanonical functions to affect tumor genome evolution through suppression or induction of polyploidization. These new findings provide a more complete mechanistic understanding of cancer progression that may, in the future, lead to novel therapeutic interventions.
Figures

References
-
- Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. - PubMed
-
- Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. - PubMed
-
- Bayani J, Selvarajah S, Maire G, Vukovic B, Al-Romaih K, Zielenska M, Squire JA. Genomic mechanisms and measurement of structural and numerical instability in cancer cells. Semin Cancer Biol. 2007;17:5–18. - PubMed
-
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. - PubMed
-
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;4260:23–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
