The mitogen-activated protein kinase p38α regulates tubular damage in murine anti-glomerular basement membrane nephritis
- PMID: 23441175
- PMCID: PMC3575386
- DOI: 10.1371/journal.pone.0056316
The mitogen-activated protein kinase p38α regulates tubular damage in murine anti-glomerular basement membrane nephritis
Abstract
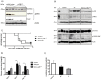
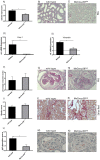
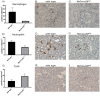
p38 mitogen-activated protein kinase (MAPK) is thought to play a central role in acute and chronic inflammatory responses. Whether p38MAPK plays a pathogenic role in crescentic GN (GN) and which of its four isoforms is preferentially involved in kidney inflammation is not definitely known. We thus examined expression and activation of p38MAPK isoforms during anti-glomerular basement membrane (GBM) nephritis. Therefore, p38α conditional knockout mice (MxCre-p38α(Δ/Δ)) were used to examine the role of p38α in anti-GBM induced nephritis. Both wild type and MxCre-p38α(Δ/Δ) mice developed acute renal failure over time. Histological examinations revealed a reduced monocyte influx and less tubular damage in MxCre-p38α(Δ/Δ) mice, whereas glomerular crescent formation and renal fibrosis was similar. Likewise, the levels of pro- and anti-inflammatory cytokines such as TNF, IL-1 and IL-10 were similar, but IL-8 was even up-regulated in MxCre-p38α(Δ/Δ) mice. In contrast, we could detect strong down-regulation of chemotactic cytokines such as CCL-2, -5 and -7, in the kidneys of MxCre-p38α(Δ/Δ) mice. In conclusion, p38α is the primary p38MAPK isoform expressed in anti-GBM nephritis and selectively affects inflammatory cell influx and tubular damage. Full protection from nephritis is however not achieved as renal failure and structural damage still occurs.
Conflict of interest statement
Figures



References
-
- Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr Opin Cell Biol 10: 205–219. - PubMed
-
- New L, Han J (1998) The p38 MAP kinase pathway and its biological function. Trends Cardiovasc Med 8: 220–228. - PubMed
-
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, et al. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183. - PubMed
-
- Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

