Common commercial and consumer products contain activators of the aryl hydrocarbon (dioxin) receptor
- PMID: 23441220
- PMCID: PMC3575475
- DOI: 10.1371/journal.pone.0056860
Common commercial and consumer products contain activators of the aryl hydrocarbon (dioxin) receptor
Abstract
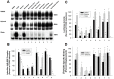
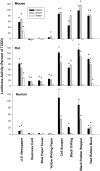
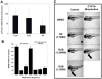
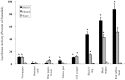
Activation of the Ah receptor (AhR) by halogenated aromatic hydrocarbons (HAHs), such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin), can produce a wide variety of toxic and biological effects. While recent studies have shown that the AhR can bind and be activated by structurally diverse chemicals, how widespread of these AhR agonists are in environmental, biological and synthetic materials remains to be determined. Using AhR-based assays, we demonstrate the presence of potent AhR agonists in a variety of common commercial and consumer items. Solvent extracts of paper, rubber and plastic products contain chemicals that can bind to and stimulate AhR DNA binding and/or AhR-dependent gene expression in hepatic cytosol, cultured cell lines, human epidermis and zebrafish embryos. In contrast to TCDD and other persistent dioxin-like HAHs, activation of AhR-dependent gene expression by these extracts was transient, suggesting that the agonists are metabolically labile. Solvent extracts of rubber products produce AhR-dependent developmental toxicity in zebrafish in vivo, and inhibition of expression of the metabolic enzyme CYP1A, significantly increased their toxic potency. Although the identity of the responsible AhR-active chemicals and their toxicological impact remain to be determined, our data demonstrate that AhR active chemicals are widely distributed in everyday products.
Conflict of interest statement
Figures

References
-
- Denison MS, Seidel SD, Rogers WJ, Ziccardi M, Winter GM, et al.. (1998) Natural and synthetic ligands for the Ah receptor. In: Puga A, Wallace KB, editors. Molecular Biology Approaches to Toxicology. Philadelphia: Taylor and Francis. pp. 393–410.
-
- Furness SGB, Whelan F (2009) The pleiotropy of dioxin toxicity – xenobiotic misappropriation of the aryl hydrocarbon receptor's alternative physiological roles. Pharmacol Ther 124: 336–353. - PubMed
-
- Safe S (1990) Polychlorinated-biphenyls (PCBs), dibenzo-para-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit Rev Toxicol 21: 51–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

