Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine
- PMID: 23442211
- PMCID: PMC3599714
- DOI: 10.1186/1878-5085-4-7
Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine
Abstract
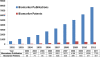
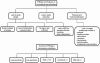
Since the emergence of the so-called omics technology, thousands of putative biomarkers have been identified and published, which have dramatically increased the opportunities for developing more effective therapeutics. These opportunities can have profound benefits for patients and for the economics of healthcare. However, the transfer of biomarkers from discovery to clinical practice is still a process filled with lots of pitfalls and limitations, mostly limited by structural and scientific factors. To become a clinically approved test, a potential biomarker should be confirmed and validated using hundreds of specimens and should be reproducible, specific and sensitive. Besides the lack of quality in biomarker validation, a number of other key issues can be identified and should be addressed. Therefore, the aim of this article is to discuss a series of interpretative and practical issues that need to be understood and resolved before potential biomarkers become a clinically approved test or are already on the diagnostic market. Some of these issues are shortly discussed here.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources