A phase II study of amrubicin as a third-line or fourth-line chemotherapy for patients with non-small cell lung cancer: Hokkaido Lung Cancer Clinical Study Group Trial (HOT) 0901
- PMID: 23442308
- PMCID: PMC3639531
- DOI: 10.1634/theoncologist.2012-0308
A phase II study of amrubicin as a third-line or fourth-line chemotherapy for patients with non-small cell lung cancer: Hokkaido Lung Cancer Clinical Study Group Trial (HOT) 0901
Abstract
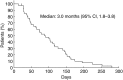
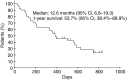
Amrubicin, a third-generation synthetic anthracycline agent, has favorable clinical activity and acceptable toxicity for the treatment of patients with non-small cell lung cancer (NSCLC) and small cell lung cancer. We conducted this study to evaluate the efficacy and safety of amrubicin for advanced NSCLC patients as a third- or fourth-line therapy. Eligible patients had recurrent or refractory advanced NSCLC after second- or third-line therapy. Patients received amrubicin, 35 mg/m(2) i.v. on days 1-3 every 3 weeks. The primary endpoint was the disease control rate (DCR). Secondary endpoints were the overall survival (OS) time, progression-free survival (PFS) time, response rate, and toxicity profile. Of the 41 patients enrolled, 26 received amrubicin as a third-line and 15 received it as a fourth-line therapy. The median number of treatment cycles was two (range, 1-9). Objective responses were complete response (n = 0), partial response (n = 4), stable disease (n = 21), progressive disease (n = 15), and not evaluable (n = 1), resulting in a DCR of 61.0% (95% confidence interval, 46.0%-75.9%). The overall response rate was 9.8% (95% confidence interval, 0.6%-18.8%). The median PFS interval was 3.0 months, median OS time was 12.6 months, and 1-year survival rate was 53.7%. Grade 3 or 4 hematological toxicities were neutropenia (68%), anemia (12%), thrombocytopenia (12%), and febrile neutropenia (17%). Nonhematological toxicities were mild and reversible. No treatment-related deaths were observed. Amrubicin showed significant clinical activity with manageable toxicities as a third- or fourth-line therapy for patients with advanced NSCLC. This study provides relevant data for routine practice and future prospective trials evaluating third- or fourth-line treatment strategies for patients with advanced NSCLC.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
A phase II study of amrubicin, a synthetic 9-aminoanthracycline, in patients with previously treated lung cancer.Lung Cancer. 2010 Jul;69(1):99-104. doi: 10.1016/j.lungcan.2009.09.012. Epub 2009 Oct 23. Lung Cancer. 2010. PMID: 19853960 Clinical Trial.
-
Phase II trial of amrubicin for second-line treatment of advanced non-small cell lung cancer: results of the West Japan Thoracic Oncology Group trial (WJTOG0401).J Thorac Oncol. 2010 Jan;5(1):105-9. doi: 10.1097/JTO.0b013e3181c07c6c. J Thorac Oncol. 2010. PMID: 19884859 Clinical Trial.
-
Multicenter phase II study of amrubicin, 9-amino-anthracycline, in patients with advanced non-small-cell lung cancer (Study 1): West Japan Thoracic Oncology Group (WJTOG) trial.Invest New Drugs. 2006 Mar;24(2):151-8. doi: 10.1007/s10637-006-5937-2. Invest New Drugs. 2006. PMID: 16502350 Clinical Trial.
-
Efficacy and Safety of Amrubicin in Non-Small-Cell Lung Cancer Patients Beyond Third-Line Therapy.Oncol Res Treat. 2019;42(1-2):52-56. doi: 10.1159/000493199. Epub 2018 Dec 12. Oncol Res Treat. 2019. PMID: 30537755
-
Amrubicin for non-small-cell lung cancer and small-cell lung cancer.Invest New Drugs. 2007 Oct;25(5):499-504. doi: 10.1007/s10637-007-9069-0. Epub 2007 Jul 13. Invest New Drugs. 2007. PMID: 17628745 Review.
Cited by
-
Recurrent pulmonary synovial sarcoma effectively treated with amrubicin: A case report.Exp Ther Med. 2015 May;9(5):1947-1949. doi: 10.3892/etm.2015.2308. Epub 2015 Feb 24. Exp Ther Med. 2015. PMID: 26136920 Free PMC article.
-
Pulmonary Large-Cell Neuroendocrine Carcinoma: From Epidemiology to Therapy.J Thorac Oncol. 2015 Aug;10(8):1133-41. doi: 10.1097/JTO.0000000000000589. J Thorac Oncol. 2015. PMID: 26039012 Free PMC article.
-
Phase II trial of S-1 as third-line or further chemotherapy in patients with advanced non-small-cell lung cancer.Int J Clin Oncol. 2014 Dec;19(6):1005-10. doi: 10.1007/s10147-014-0663-9. Epub 2014 Feb 18. Int J Clin Oncol. 2014. PMID: 24532162 Clinical Trial.
-
A single-arm phase II trial of weekly nanoparticle albumin-bound paclitaxel (nab-paclitaxel) monotherapy after standard of chemotherapy for previously treated advanced non-small cell lung cancer.Cancer Chemother Pharmacol. 2019 Aug;84(2):351-358. doi: 10.1007/s00280-019-03843-0. Epub 2019 Apr 16. Cancer Chemother Pharmacol. 2019. PMID: 30993397 Free PMC article. Clinical Trial.
-
Phase II study of amrubicin (SM-5887), a synthetic 9-aminoanthracycline, as first line treatment in patients with metastatic or unresectable soft tissue sarcoma: durable response in myxoid liposarcoma with TLS-CHOP translocation.Invest New Drugs. 2016 Apr;34(2):243-52. doi: 10.1007/s10637-016-0333-z. Epub 2016 Feb 20. Invest New Drugs. 2016. PMID: 26897615 Clinical Trial.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J Clin Oncol. 2004;22:330–353. - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources