HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab
- PMID: 23442322
- PMCID: PMC3600586
- DOI: 10.1158/0008-5472.CAN-12-3349
HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab
Abstract
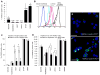
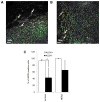
Although current breast cancer treatment guidelines limit the use of HER2-blocking agents to tumors with HER2 gene amplification, recent retrospective analyses suggest that a wider group of patients may benefit from this therapy. Using breast cancer cell lines, mouse xenograft models and matched human primary and metastatic tissues, we show that HER2 is selectively expressed in and regulates self-renewal of the cancer stem cell (CSC) population in estrogen receptor-positive (ER(+)), HER2(-) luminal breast cancers. Although trastuzumab had no effects on the growth of established luminal breast cancer mouse xenografts, administration after tumor inoculation blocked subsequent tumor growth. HER2 expression is increased in luminal tumors grown in mouse bone xenografts, as well as in bone metastases from patients with breast cancer as compared with matched primary tumors. Furthermore, this increase in HER2 protein expression was not due to gene amplification but rather was mediated by receptor activator of NF-κB (RANK)-ligand in the bone microenvironment. These studies suggest that the clinical efficacy of adjuvant trastuzumab may relate to the ability of this agent to target the CSC population in a process that does not require HER2 gene amplification. Furthermore, these studies support a CSC model in which maximal clinical benefit is achieved when CSC targeting agents are administered in the adjuvant setting. Cancer Res; 73(5); 1635-46. ©2012 AACR.
©2012 AACR.
Conflict of interest statement
M. S. Wicha has financial holdings in OncoMed Pharmaceuticals, receives research support from Dompe and MedImmune, serves on the scientific advisory board of VERISTEM.
H. Korkaya receives research support from MedImmune.
D. F. Hayes has received research support from Pfizer, Novartis and Veridex and holds stock option for his role on the scientific advisory board for OncImmune.
Figures





References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First-line Herceptin monotherapy in metastatic breast cancer. Oncology. 2001;61 (Suppl 2):37–42. - PubMed
-
- Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

