Tumor-induced endothelial cell surface heterogeneity directly affects endothelial cell escape from a cell-mediated immune response in vitro
- PMID: 23442592
- PMCID: PMC3667939
- DOI: 10.4161/hv.22828
Tumor-induced endothelial cell surface heterogeneity directly affects endothelial cell escape from a cell-mediated immune response in vitro
Abstract
Immune-mediated damage to tumor vessels is a potential means of preventing solid tumor progression. Antiangiogenic cancer vaccines capable of inducing this kind of damage include formulations comprised of endothelial cell-specific antigens. Identification of antigens capable of eliciting efficient vaccination is difficult because the endothelial cell phenotype is affected by surrounding tissues, including angiogenic stimuli received from surrounding tumor cells. Therefore, phenotype endothelial cell variations (heterogeneity) were examined in the context of the development of an efficient vaccine using mass spectrometry-based cell surface profiling. This approach was applied to primary human microvascular endothelial cell (HMEC) cultures proliferated under growth stimuli provided by either normal tissues (growth supplement from human hypothalamus) or cancer cells (MCF-7, LNCap and HepG2). It was found that tumors induced pronounced, tumor type-dependent changes to HMEC surface targets that in an in vitro model of human antiangiogenic vaccination directly facilitated HMEC escape from cytotoxic T cell-mediated cell death. Furthermore, it was found that tumors influenced the HMEC phenotype unidirectionally and that HMEC imunogenicity was reciprocal to the intensity of tumor-induced changes to the HMEC surface. These findings provide data for the design of tumor-specific endothelial cell based vaccines with sufficient immunogenicity without posing a risk to the elicitation of autoimmunity if administered in vivo.
Keywords: antiangiogenic cancer vaccine; cell heterogeneity; cell proteomic footprinting; cell surface profiling; microvascular endothelial cells; vaccine design.
Figures
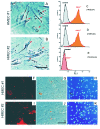
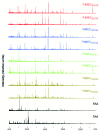


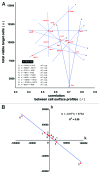
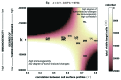
References
-
- Bussolino F, Arese M, Audero E, Giraudo E, Marchiò S, Mitola S, et al. Biological aspects of tumour angiogenesis. Cancer Modelling and Simulation. London: Chapman and Hall/CRC; 2003. p. 1–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
