Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children
- PMID: 23442823
- PMCID: PMC3903909
- DOI: 10.4161/hv.23087
Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children
Abstract
Japanese encephalitis chimeric virus vaccine (JE-CV) is a licensed vaccine indicated in a single dose administration for primary immunization. This controlled phase III comparative trial enrolled children aged 36-42 mo in the Philippines. 345 children who had received one dose of JE-CV in a study two years earlier, received a JE-CV booster dose. 105 JE-vaccine-naïve children in general good health were randomized to receive JE-CV (JE-vaccine naïve group; 46 children) or varicella vaccine (safety control group; 59 children). JE neutralizing antibody titers were assessed using PRNT50. Immunological memory was observed in children who had received the primary dose of JE-CV before. Seven days after the JE-CV booster dose administration, 96.2% and 66.8% of children were seroprotected and had seroconverted, respectively, and the geometric mean titer (GMT) was 231 1/dil. Twenty-eight days after the JE-CV booster dose seroprotection and seroconversion were achieved in 100% and 95.3% of children, respectively, and the GMT was 2,242 1/dil. In contrast, only 15.4% of JE-CV-vaccine naïve children who had not received any prior JE vaccine were seroprotected seven days after they received JE-CV. One year after receiving the JE-CV booster dose, 99.4% of children remained seroprotected. We conclude that JE-CV is effective and safe, both as a single dose and when administrated as a booster dose. A booster dose increases the peak GMT above the peak level reached after primary immunization and the antibody persistence is maintained at least one year after the JE-CV booster dose administration. Five year follow up is ongoing.
Keywords: Japanese encephalitis vaccines; active immunization; attenuated vaccines; booster immunization; humoral immune response.
Figures

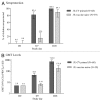
References
-
- Halstead SB, Jacobson J. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines, 5th ed. Philadelphia (PA): Saunders Elsevier, 2008:311–52.
-
- Fischer M, Lindsey N, Staples JE, Hills S, Centers for Disease Control and Prevention (CDC) Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-1):1–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
