Modeling the flexural rigidity of rod photoreceptors
- PMID: 23442852
- PMCID: PMC3552261
- DOI: 10.1016/j.bpj.2012.11.3835
Modeling the flexural rigidity of rod photoreceptors
Abstract
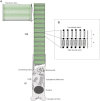
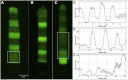
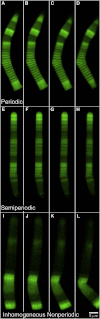
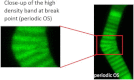
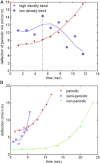
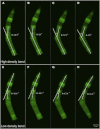
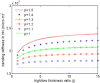
In vertebrate eyes, the rod photoreceptor has a modified cilium with an extended cylindrical structure specialized for phototransduction called the outer segment (OS). The OS has numerous stacked membrane disks and can bend or break when subjected to mechanical forces. The OS exhibits axial structural variation, with extended bands composed of a few hundred membrane disks whose thickness is diurnally modulated. Using high-resolution confocal microscopy, we have observed OS flexing and disruption in live transgenic Xenopus rods. Based on the experimental observations, we introduce a coarse-grained model of OS mechanical rigidity using elasticity theory, representing the axial OS banding explicitly via a spring-bead model. We calculate a bending stiffness of ∼10(5) nN⋅μm2, which is seven orders-of-magnitude larger than that of typical cilia and flagella. This bending stiffness has a quadratic relation to OS radius, so that thinner OS have lower fragility. Furthermore, we find that increasing the spatial frequency of axial OS banding decreases OS rigidity, reducing its fragility. Moreover, the model predicts a tendency for OS to break in bands with higher spring number density, analogous to the experimental observation that transgenic rods tended to break preferentially in bands of high fluorescence. We discuss how pathological alterations of disk membrane properties by mutant proteins may lead to increased OS rigidity and thus increased breakage, ultimately contributing to retinal degeneration.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Will the rod bend or break? Analyzing the structural resilience of cellular organelles.Biophys J. 2013 Jan 22;104(2):284-5. doi: 10.1016/j.bpj.2012.12.023. Biophys J. 2013. PMID: 23442849 Free PMC article. No abstract available.
References
-
- Dowling J.E. Belknap Press/Harvard University Press; Cambridge, MA: 1987. The Retina: An Approachable Part of the Brain. 12–42.
-
- Pugh E.N., Jr., Lamb T.D. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga D.G., de Grip W.J., Pugh E.N. Jr., editors. Handbook of Biological Physics. Elsevier; Amsterdam: 2000. pp. 183–254.
-
- Papermaster D.S., Dreyer W.J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13:2438–2444. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

