Nicotinic receptor transduction zone: invariant arginine couples to multiple electron-rich residues
- PMID: 23442857
- PMCID: PMC3552258
- DOI: 10.1016/j.bpj.2012.12.013
Nicotinic receptor transduction zone: invariant arginine couples to multiple electron-rich residues
Abstract
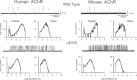
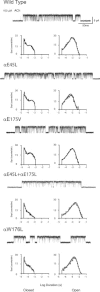
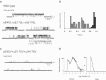
Gating of the muscle-type acetylcholine receptor (AChR) channel depends on communication between the ACh-binding site and the remote ion channel. A key region for this communication is located within the structural transition zone between the ligand-binding and pore domains. Here, stemming from β-strand 10 of the binding domain, the invariant αArg209 lodges within the hydrophobic interior of the subunit and is essential for rapid and efficient channel gating. Previous charge-reversal experiments showed that the contribution of αArg209 to channel gating depends strongly on αGlu45, also within this region. Here we determine whether the contribution of αArg209 to channel gating depends on additional anionic or electron-rich residues in this region. Also, to reconcile diverging findings in the literature, we compare the dependence of αArg209 on αGlu45 in AChRs from different species, and compare the full agonist ACh with the weak agonist choline. Our findings reveal that the contribution of αArg209 to channel gating depends on additional nearby electron-rich residues, consistent with both electrostatic and steric contributions. Furthermore, αArg209 and αGlu45 show a strong interdependence in both human and mouse AChRs, whereas the functional consequences of the mutation αE45R depend on the agonist. The emerging picture shows a multifaceted network of interdependent residues that are required for communication between the ligand-binding and pore domains.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
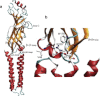


References
-
- Sine S.M. The nicotinic receptor ligand binding domain. J. Neurobiol. 2002;53:431–446. - PubMed
-
- Taly A., Corringer P.J., Changeux J.P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009;8:733–750. - PubMed
-
- Celie P.H., van Rossum-Fikkert S.E., Sixma T.K. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. - PubMed
-
- Gao F., Bren N., Sine S.M. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. J. Biol. Chem. 2005;280:8443–8451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

