Collective diffusion model for ion conduction through microscopic channels
- PMID: 23442858
- PMCID: PMC3552282
- DOI: 10.1016/j.bpj.2012.11.3826
Collective diffusion model for ion conduction through microscopic channels
Abstract
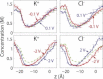
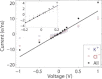
Ion conduction through microscopic channels is of central importance in both biology and nanotechnology. To better understand the current-voltage (I-V) dependence of ion channels, here we describe and prove a collective diffusion model that quantitatively relates the spontaneous ion permeation at equilibrium to the stationary ionic fluxes driven by small voltages. The model makes it possible to determine the channel conductance in the linear I-V range from equilibrium simulations without the application of a voltage. To validate the theory, we perform molecular-dynamics simulations on two channels-a conical-shaped nanopore and the transmembrane pore of an α-hemolysin-under both equilibrium and nonequilibrium conditions. The simulations reveal substantial couplings between the motions of cations and anions, which are effectively captured by the collective coordinate in the model. Although the two channels exhibit very different linear ranges in the I-V curves, in both cases the channel conductance at small voltages is in reasonable agreement with the prediction from the equilibrium simulation. The simulations also suggest that channel charges, rather than geometric asymmetry, play a more prominent role in current rectification.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Mechanosensitive channel MscS in the open state: modeling of the transition, explicit simulations, and experimental measurements of conductance.J Gen Physiol. 2008 Jul;132(1):67-83. doi: 10.1085/jgp.200810000. J Gen Physiol. 2008. PMID: 18591417 Free PMC article.
-
Ion permeation through the alpha-hemolysin channel: theoretical studies based on Brownian dynamics and Poisson-Nernst-Plank electrodiffusion theory.Biophys J. 2004 Oct;87(4):2299-309. doi: 10.1529/biophysj.104.044008. Biophys J. 2004. PMID: 15454431 Free PMC article.
-
Voltage-dependent hydration and conduction properties of the hydrophobic pore of the mechanosensitive channel of small conductance.Biophys J. 2006 May 15;90(10):3555-69. doi: 10.1529/biophysj.105.080432. Epub 2006 Feb 24. Biophys J. 2006. PMID: 16500980 Free PMC article.
-
Molecular simulation studies of hydrophobic gating in nanopores and ion channels.Biochem Soc Trans. 2015 Apr;43(2):146-50. doi: 10.1042/BST20140256. Biochem Soc Trans. 2015. PMID: 25849908 Review.
-
Asymmetric ion transport through ion-channel-mimetic solid-state nanopores.Acc Chem Res. 2013 Dec 17;46(12):2834-46. doi: 10.1021/ar400024p. Epub 2013 May 28. Acc Chem Res. 2013. PMID: 23713693 Review.
Cited by
-
Autonomous pump against concentration gradient.Sci Rep. 2016 Mar 21;6:23414. doi: 10.1038/srep23414. Sci Rep. 2016. PMID: 26996204 Free PMC article.
-
Dissecting current rectification through asymmetric nanopores.Biophys J. 2025 Feb 18;124(4):597-603. doi: 10.1016/j.bpj.2024.11.3318. Epub 2024 Nov 29. Biophys J. 2025. PMID: 39614613
-
Changes in Ion Selectivity Following the Asymmetrical Addition of Charge to the Selectivity Filter of Bacterial Sodium Channels.Entropy (Basel). 2020 Dec 9;22(12):1390. doi: 10.3390/e22121390. Entropy (Basel). 2020. PMID: 33316962 Free PMC article.
-
Ionic transport through a protein nanopore: a Coarse-Grained Molecular Dynamics Study.Sci Rep. 2019 Oct 31;9(1):15740. doi: 10.1038/s41598-019-51942-y. Sci Rep. 2019. PMID: 31673049 Free PMC article.
References
-
- Hille B. Sinauer; Sunderland, MA: 2001. Ion Channels of Excitable Membranes.
-
- Roux B., Allen T., Im W. Theoretical and computational models of biological ion channels. Q. Rev. Biophys. 2004;37:15–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

