An anionic phospholipid enables the hydrophobic surfactant proteins to alter spontaneous curvature
- PMID: 23442910
- PMCID: PMC3566453
- DOI: 10.1016/j.bpj.2012.12.041
An anionic phospholipid enables the hydrophobic surfactant proteins to alter spontaneous curvature
Abstract
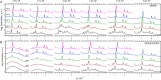
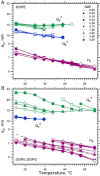
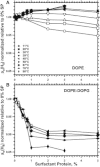
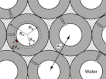
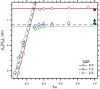
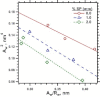
The hydrophobic surfactant proteins, SP-B and SP-C, greatly accelerate the adsorption of the surfactant lipids to an air/water interface. Previous studies of factors that affect curvature suggest that vesicles may adsorb via a rate-limiting structure with prominent negative curvature, in which the hydrophilic face of the lipid leaflets is concave. To determine if SP-B and SP-C might promote adsorption by inducing negative curvature, we used small-angle x-ray scattering to test whether the physiological mixture of the two proteins affects the radius of cylindrical monolayers in the inverse hexagonal phase. With dioleoyl phosphatidylethanolamine alone, the proteins had no effect on the hexagonal lattice constant, suggesting that the proteins fail to insert into the cylindrical monolayers. The surfactant lipids also contain ∼10% anionic phospholipids, which might allow incorporation of the cationic proteins. With 10% of the anionic dioleoyl phosphatidylglycerol added to dioleoyl phosphatidylethanolamine, the proteins induced a dose-related decrease in the hexagonal lattice constant. At 30°C, the reduction reached a maximum of 8% relative to the lipids alone at ∼1% (w/w) protein. Variation of NaCl concentration tested whether the effect of the protein represented a strictly electrostatic effect that screening by electrolyte would eliminate. With concentrations up to 3 M NaCl, the dose-related change in the hexagonal lattice constant decreased but persisted. Measurements at different hydrations determined the location of the pivotal plane and proved that the change in the lattice constant produced by the proteins resulted from a shift in spontaneous curvature. These results provide the most direct evidence yet that the surfactant proteins can induce negative curvature in lipid leaflets. This finding supports the model in which the proteins promote adsorption by facilitating the formation of a negatively curved, rate-limiting structure.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Hydrophobic surfactant proteins strongly induce negative curvature.Biophys J. 2015 Jul 7;109(1):95-105. doi: 10.1016/j.bpj.2015.05.030. Biophys J. 2015. PMID: 26153706 Free PMC article.
-
Effects of spontaneous curvature on interfacial adsorption and collapse of phospholipid monolayers.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L876-L882. doi: 10.1152/ajplung.00193.2024. Epub 2024 Oct 22. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39437761
-
Hydrophobic surfactant proteins induce a phosphatidylethanolamine to form cubic phases.Biophys J. 2010 Apr 21;98(8):1549-57. doi: 10.1016/j.bpj.2009.12.4302. Biophys J. 2010. PMID: 20409474 Free PMC article.
-
Protein-lipid interactions and surface activity in the pulmonary surfactant system.Chem Phys Lipids. 2006 Jun;141(1-2):105-18. doi: 10.1016/j.chemphyslip.2006.02.017. Epub 2006 Mar 20. Chem Phys Lipids. 2006. PMID: 16600200 Review.
-
Hydrophobic surfactant proteins and their analogues.Neonatology. 2007;91(4):303-10. doi: 10.1159/000101346. Epub 2007 Jun 7. Neonatology. 2007. PMID: 17575474 Review.
Cited by
-
Lipid-Protein and Protein-Protein Interactions in the Pulmonary Surfactant System and Their Role in Lung Homeostasis.Int J Mol Sci. 2020 May 25;21(10):3708. doi: 10.3390/ijms21103708. Int J Mol Sci. 2020. PMID: 32466119 Free PMC article. Review.
-
Hydrophobic surfactant proteins strongly induce negative curvature.Biophys J. 2015 Jul 7;109(1):95-105. doi: 10.1016/j.bpj.2015.05.030. Biophys J. 2015. PMID: 26153706 Free PMC article.
-
Techniques to evaluate surfactant activity for a personalized therapy of RDS neonates.Biomed J. 2021 Dec;44(6):671-677. doi: 10.1016/j.bj.2021.11.001. Epub 2021 Nov 7. Biomed J. 2021. PMID: 34758409 Free PMC article. Review.
-
Effects of spontaneous curvature on interfacial adsorption and collapse of phospholipid monolayers.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L876-L882. doi: 10.1152/ajplung.00193.2024. Epub 2024 Oct 22. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39437761
-
Divide & Conquer: Surfactant Protein SP-C and Cholesterol Modulate Phase Segregation in Lung Surfactant.Biophys J. 2017 Aug 22;113(4):847-859. doi: 10.1016/j.bpj.2017.06.059. Biophys J. 2017. PMID: 28834721 Free PMC article.
References
-
- Lachmann B., Grossmann G., Robertson B. Lung mechanics during spontaneous ventilation in premature and fullterm rabbit neonates. Respir. Physiol. 1979;38:283–302. - PubMed
-
- Clements J.A. Functions of the alveolar lining. Am. Rev. Respir. Dis. 1977;115(6 II):67–71. - PubMed
-
- Sen A., Hui S.-W., Egan E.A. Localization of lipid exchange sites between bulk lung surfactants and surface monolayer: freeze fracture study. J. Colloid Interface Sci. 1988;126:355–360.
-
- Schürch S., Schürch D., Robertson B. Surface activity of lipid extract surfactant in relation to film area compression and collapse. J. Appl. Physiol. 1994;77:974–986. - PubMed
-
- Haller T., Dietl P., Putz G. Tracing surfactant transformation from cellular release to insertion into an air-liquid interface. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L1009–L1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

