Effects of sphingomyelin headgroup size on interactions with ceramide
- PMID: 23442911
- PMCID: PMC3566447
- DOI: 10.1016/j.bpj.2012.12.026
Effects of sphingomyelin headgroup size on interactions with ceramide
Abstract
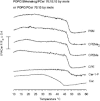
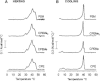
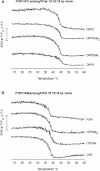
Sphingomyelins (SMs) and ceramides are known to interact favorably in bilayer membranes. Because ceramide lacks a headgroup that could shield its hydrophobic body from unfavorable interactions with water, accommodation of ceramide under the larger phosphocholine headgroup of SM could contribute to their favorable interactions. To elucidate the role of SM headgroup for SM/ceramide interactions, we explored the effects of reducing the size of the phosphocholine headgroup (removing one, two, or three methyls on the choline moiety, or the choline moiety itself). Using differential scanning calorimetry and fluorescence spectroscopy, we found that the size of the SM headgroup had no marked effect on the thermal stability of ordered domains formed by SM analog/palmitoyl ceramide (PCer) interactions. In more complex bilayers composed of a fluid glycerophospholipid, SM analog, and PCer, the thermal stability and molecular order of the laterally segregated gel domains were roughly identical despite variation in SM headgroup size. We suggest that that the association between PCer and SM analogs was stabilized by ceramide's aversion for disordered phospholipids, by interfacial hydrogen bonding between PCer and the SM analogs, and by attractive van der Waals' forces between saturated chains of PCer and SM analogs.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The Influence of Hydrogen Bonding on Sphingomyelin/Colipid Interactions in Bilayer Membranes.Biophys J. 2016 Jan 19;110(2):431-440. doi: 10.1016/j.bpj.2015.11.3515. Biophys J. 2016. PMID: 26789766 Free PMC article.
-
Palmitoyl ceramide promotes milk sphingomyelin gel phase domains formation and affects the mechanical properties of the fluid phase in milk-SM/DOPC supported membranes.Biochim Biophys Acta Biomembr. 2018 Mar;1860(3):635-644. doi: 10.1016/j.bbamem.2017.12.005. Epub 2017 Dec 8. Biochim Biophys Acta Biomembr. 2018. PMID: 29229528
-
Homogeneous and Heterogeneous Bilayers of Ternary Lipid Compositions Containing Equimolar Ceramide and Cholesterol.Langmuir. 2019 Apr 16;35(15):5305-5315. doi: 10.1021/acs.langmuir.9b00324. Epub 2019 Apr 8. Langmuir. 2019. PMID: 30924341
-
Cholesterol interactions with ceramide and sphingomyelin.Chem Phys Lipids. 2016 Sep;199:26-34. doi: 10.1016/j.chemphyslip.2016.04.002. Epub 2016 Apr 27. Chem Phys Lipids. 2016. PMID: 27132117 Review.
-
The importance of hydrogen bonding in sphingomyelin's membrane interactions with co-lipids.Biochim Biophys Acta. 2016 Feb;1858(2):304-10. doi: 10.1016/j.bbamem.2015.12.008. Epub 2015 Dec 4. Biochim Biophys Acta. 2016. PMID: 26656158 Review.
Cited by
-
Natural Ceramides and Lysophospholipids Cosegregate in Fluid Phosphatidylcholine Bilayers.Biophys J. 2019 Mar 19;116(6):1105-1114. doi: 10.1016/j.bpj.2019.02.002. Epub 2019 Feb 10. Biophys J. 2019. PMID: 30795873 Free PMC article.
-
Bilayer Interactions among Unsaturated Phospholipids, Sterols, and Ceramide.Biophys J. 2017 Apr 25;112(8):1673-1681. doi: 10.1016/j.bpj.2017.03.016. Biophys J. 2017. PMID: 28445758 Free PMC article.
-
Influence of Hydroxylation, Chain Length, and Chain Unsaturation on Bilayer Properties of Ceramides.Biophys J. 2015 Oct 20;109(8):1639-51. doi: 10.1016/j.bpj.2015.08.040. Biophys J. 2015. PMID: 26488655 Free PMC article.
-
Current Methods for Detecting Cell Membrane Transient Interactions.Front Chem. 2020 Dec 7;8:603259. doi: 10.3389/fchem.2020.603259. eCollection 2020. Front Chem. 2020. PMID: 33365301 Free PMC article. Review.
-
Sphingomyelinase-Mediated Multitimescale Clustering of Ganglioside GM1 in Heterogeneous Lipid Membranes.Adv Sci (Weinh). 2021 Oct;8(20):e2101766. doi: 10.1002/advs.202101766. Epub 2021 Sep 2. Adv Sci (Weinh). 2021. PMID: 34473415 Free PMC article.
References
-
- Zwaal R.F., Roelofsen B., van Deenen L.L. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim. Biophys. Acta. 1975;406:83–96. - PubMed
-
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. - PubMed
-
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. - PubMed
-
- Pike L.J. Rafts defined: a report on the Keystone Symposium on lipid rafts and cell function. J. Lipid Res. 2006;47:1597–1598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

